Enhancing the Performance of the Electrocoagulation−Filtration System Treating Mariculture Tailwaters by Using Alternating Pulse Current: Effects of Current Density and Current Conversion Period
Abstract
:1. Introduction
2. Materials and Methods
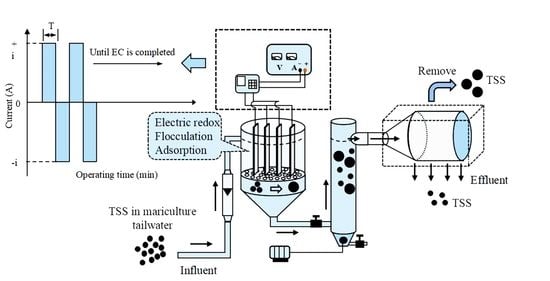
2.1. Experimental Set-Up
2.2. Experimental Water
2.3. Experimental Design
2.4. Analysis Method
3. Results and Discussion
3.1. Effects of Current Density and CCP on Electrode Mass and Al Dissolution
3.2. Effects of Current Density and CCP on TSS Removal
3.3. Effects of Current Density and CCP on CODMn Removal
3.4. Effects of Current Density and CCP on TN Removal
3.5. Effects of Current Density and CCP on Energy Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Umembamalu, C.J. Electrocoagulation-flocculation of aquaculture effluent using hybrid iron and aluminium electrodes: A comparative study. Chem. Eng. J. Adv. 2021, 6, 100107. [Google Scholar] [CrossRef]
- Xu, J.P.; Du, Y.S.; Qiu, T.L.; Zhou, L.; Li, Y.; Chen, F.D.; Sun, J.M. Application of hybrid electrocoagulation-filtration methods in the pretreatment of marine aquaculture wastewater. Water Sci. Technol. 2021, 83, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Heffron, J.; McDermid, B.; Maher, E.; McNamara, P.J.; Mayer, B.K. Mechanisms of virus mitigation and suitability of bacteriophages as surrogates in drinking water treatment by iron electrocoagulation. Water Res. 2019, 163, 114877. [Google Scholar] [CrossRef]
- García-Morales, M.A.; Juárez, J.C.G.; Martínez-Gallegos, S.; Roa-Morales, G.; Peralta, E.; del Campo López, E.M.; Barrera-Díaz, C.; Miranda, V.M.; Blancas, T.T. Pretreatment of real wastewater from the chocolate manufacturing industry through an integrated process of electrocoagulation and sand filtration. Int. J. Photoenergy 2018, 2018, 2146751. [Google Scholar] [CrossRef] [Green Version]
- Khorram, A.G.; Fallah, N. Comparison of electrocoagulation and photocatalytic process for treatment of industrial dyeing wastewater: Energy consumption analysis. Environ. Prog. Sustain. Energy 2020, 39, 13288. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Pulliainen, M.; Sillanpää, M. Effect of electrochemical cell structure on natural organic matter (NOM) removal from surface water through electrocoagulation (EC). Sep. Purif. Technol. 2012, 99, 20–27. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, G.; Cheng, G.; Wang, Y.; Fu, H. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to reverse osmosis membranes. Desalination 2014, 344, 454–462. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, B.K. Assessment of kinetics behavior of electrocoagulation process for the removal of suspended solids and metals from synthetic water. Environ. Eng. Res. 2017, 22, 150–153. [Google Scholar] [CrossRef]
- Chellam, S.; Sari, M.A. Aluminum electrocoagulation as pretreatment during microfiltration of surface water containing NOM: A review of fouling, NOM, DBP, and virus control. J. Hazard. Mater. 2016, 304, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.Q.; Graham, N.; André, C.; Kelsall, G.H.; Brandon, N. Laboratory study of electro-coagulation-flotation for water treatment. Water Res. 2002, 36, 4064–4078. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Graham, N.J.D.; André, C.M.; Kelsall, G.H.; Brandon, N.P.; Chipps, M.J. Comparative performance of an electrocoagulation/flotation system with chemical coagulation/dissolved air flotation: A pilot-scale trial. Water Sci. Technol. Water Supply 2002, 2, 289–297. [Google Scholar] [CrossRef]
- Sari, M.A.; Chellam, S. Electrocoagulation process considerations during advanced pretreatment for brackish inland surface water desalination: Nanofilter fouling control and permeate water quality. Desalination 2017, 410, 66–76. [Google Scholar] [CrossRef]
- Xie, S.; Yuan, S.; Liao, P.; Tong, M.; Gan, Y.; Wang, Y. Iron-anode enhanced sand filter for arsenic removal from tube well water. Environ. Sci. Technol. 2017, 51, 889–896. [Google Scholar] [CrossRef]
- Bian, Y.; Ge, Z.; Albano, C.; Lobo, F.L.; Ren, Z.J. Oily bilge water treatment using DC/AC powered electrocoagulation. Environ. Sci. Water Res. Technol. 2019, 5, 1654–1660. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P. Electrode Passivation, Faradaic Efficiency, and Performance Enhancement Strategies in Electrocoagulation-A Review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef]
- Yang, Z.H.; Xu, H.Y.; Zeng, G.M.; Luo, Y.L.; Yang, X.; Huang, J.; Wang, L.K.; Song, P.P. The behavior of dissolution/passivation and the transformation of passive films during electrocoagulation: Influences of initial pH, Cr (VI) concentration, and alternating pulsed current. Electrochim. Acta 2015, 153, 149–158. [Google Scholar] [CrossRef]
- Gabrielli, C.; Maurin, G.; Francy-Chausson, H.; Thery, P.; Tran, T.T.M.; Tlili, M. Electrochemical water softening: Principle and application. Desalination 2016, 201, 150–163. [Google Scholar] [CrossRef]
- Trompette, J.L.; Vergnes, H. On the crucial influence of some supporting electrolytes during electrocoagulation in the presence of aluminum electrodes. J. Hazard. Mater. 2009, 163, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Belkada, R.; Miroud, D. Simultaneous removal of chromium (VI) and fluoride by electrocoagulation–electroflotation: Application of a hybrid Fe-Al anode. Chem. Eng. J. 2015, 267, 153–162. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, F.; Wei, N.; Yang, C.; Yang, J.; Wu, Y.; Li, Y.; Xu, K.Q.; Chen, X.C.; Zhang, C.P. Comparison of Cr (VI) removal by direct and pulse current electrocoagulation: Implications for energy consumption optimization, sludge reduction and floc magnetism. J. Water Process Eng. 2020, 37, 101387. [Google Scholar] [CrossRef]
- Eyvaz, M.; Kirlaroglu, M.; Aktas, T.S.; Yuksel, E. The effects of alternating current electrocoagulation on dye removal from aqueous solutions. Chem. Eng. J. 2009, 153, 16–22. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, T.; Chen, F.; Zhou, L.; Sun, J.; Du, Y. Construction and application of an electrocoagulation and filtration linkage control system in a recirculating aquaculture system. J. Water Process Eng. 2021, 44, 102379. [Google Scholar] [CrossRef]
- Hu, C.; Sun, J.; Wang, S.; Liu, R.; Liu, H.; Qu, J. Enhanced efficiency in HA removal by electrocoagulation through optimizing flocs properties: Role of current density and pH. Sep. Purif. Technol. 2017, 175, 248–254. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater. American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Zhen, Z.; Yao, J.; Pang, Z.; Bo, L. Optimization of electrocoagulation process to eliminate CODMn in micro-polluted surface water using response surface method. J. Dispers. Sci. Technol. 2016, 37, 743–751. [Google Scholar] [CrossRef]
- Müller, S.; Behrends, T.; van Genuchten, C.M. Sustaining efficient production of aqueous iron during repeated operation of Fe(0)-electrocoagulation. Water Res. 2019, 155, 455–464. [Google Scholar] [CrossRef]
- Eyvaz, M.; Gürbulak, E.; Kara, S.; Yüksel, E. Preventing of cathode passivation/deposition in electrochemical treatment methods–a case study on winery wastewater with electrocoagulation. In Modern Electrochemical Methods in Nano, Surface and Corrosion Science; IntechOpen: London, UK, 2014; Volume 1. [Google Scholar] [CrossRef] [Green Version]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Vasudevan, S.; Kannan, B.S.; Lakshmi, J.; Mohanraj, S.; Sozhan, G. Effects of alternating and direct current in electrocoagulation process on the removal of fluoride from water. J. Chem. Technol. Biotechnol. 2011, 86, 428–436. [Google Scholar] [CrossRef]
- Mao, X.; Hong, S.; Zhu, H.; Lin, H.; Wei, L.; Gan, F. Alternating pulse current in electrocoagulation for wastewater treatment to prevent the passivation of al electrode. J. Wuhan Univ. Technol.-Mater Sci. Ed. 2008, 23, 239–241. [Google Scholar] [CrossRef]
- Chen, M.; Dollar, O.; Shafer-Peltier, K.E.; Randtke, S.; Peltier, E. Boron removal by electrocoagulation: Removal mechanism, adsorption models and factors influencing removal. Water Res. 2020, 170, 115362. [Google Scholar] [CrossRef]
- Ashraf, S.N.; Rajapakse, J.; Dawes, L.A.; Millar, G.J. Impact of turbidity, hydraulic retention time, and polarity reversal upon iron electrode based electrocoagulation pre-treatment of coal seam gas associated water. Environ. Technol. Innov. 2021, 23, 101622. [Google Scholar] [CrossRef]
- Pi, K.W.; Xiao, Q.; Zhang, H.Q.; Xia, M.; Gerson, A.R. Decolorization of synthetic methyl orange wastewater by electrocoagulation with periodic reversal of electrodes and optimization by RSM. Process Saf. Environ. Prot. 2014, 92, 796–806. [Google Scholar] [CrossRef]
- Eyvaz, M. Treatment of brewery wastewater with electrocoagulation: Improving the process performance by using alternating pulse current. Int. J. Electrochem. Sci. 2016, 11, 4988–5008. [Google Scholar] [CrossRef]
- Govindan, K.; Angelin, A.; Rangarajan, M. Critical evaluation of mechanism responsible for biomass abatement during electrochemical coagulation (EC) process: A critical review. J. Environ. Manag. 2018, 227, 335–353. [Google Scholar] [CrossRef]
- Kamyar, S.; John, A.; Yu-Hsuan, C.; Siavash, D.; Mohanad, K.; Ranil, W.S. Electrocoagulation followed by ultrafiltration for treating poultry processing wastewater. J. Environ. Chem. Eng. 2018, 6, 4937–4944. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Q.; Xu, X.; Cao, G.; He, C.; Wang, Y.; Yang, M. Simultaneous removal of Zn2+ and Mn2+ ions from synthetic and real smelting wastewater using electrocoagulation process: Influence of pulse current parameters and anions. Sep. Purif. Technol. 2017, 188, 316–328. [Google Scholar] [CrossRef]
- Govindan, K.; Noel, M.; Mohan, R. Removal of nitrate ion from water by electrochemical approaches. J. Water Process Eng. 2015, 6, 58–63. [Google Scholar] [CrossRef]
- Karamati-Niaragh, E.; Moghaddam, M.; Emamjomeh, M.M.; Nazlabadi, E. Evaluation of direct and alternating current on nitrate removal using a continuous electrocoagulation process: Economical and environmental approaches through RSM. J. Environ. Manag. 2018, 230, 245–254. [Google Scholar] [CrossRef]






| Current Type | CCP (min) | j = 15 A/m2 | j = 30 A/m2 | j = 45 A/m2 | |||
|---|---|---|---|---|---|---|---|
| U (V) | I (A) | U (V) | I (A) | U (V) | I (A) | ||
| DC | 0 | 0.94 | 0.36 | 1.03 | 0.71 | 1.20 | 1.08 |
| APC | 1 | 0.94 | 0.36 | 1.01 | 0.71 | 1.10 | 1.08 |
| 5 | 0.94 | 0.36 | 1.01 | 0.71 | 1.13 | 1.08 | |
| 10 | 0.94 | 0.36 | 1.02 | 0.71 | 1.15 | 1.08 | |
| Current Type | CCP (min) | j = 15 A/m2 | j = 30 A/m2 | j = 45 A/m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTheoretical Al (mg/L) | CActual Al (mg/L) | Φ (%) | CTheoretical Al (mg/L) | CActual Al (mg/L) | Φ (%) | CTheoretical Al (mg/L) | CActual Al (mg/L) | Φ (%) | ||
| DC | 0 | 1.21 | 1.54 | 127 | 2.38 | 2.93 | 123 | 3.63 | 4.21 | 116 |
| APC | 1 | 1.21 | 1.77 | 146 | 2.38 | 3.42 | 144 | 3.63 | 5.15 | 142 |
| 5 | 1.21 | 1.69 | 140 | 2.38 | 3.27 | 137 | 3.63 | 4.83 | 133 | |
| 10 | 1.21 | 1.60 | 132 | 2.38 | 3.07 | 129 | 3.63 | 4.50 | 124 | |
| Current Type | j = 15 A/m2 | j = 30 A/m2 | j = 45 A/m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TMDF (min) | mMDF (mg) | CActual Al (mg/L) | TMDF (min) | mMDF (mg) | CActual Al (mg/L) | TMDF (min) | mMDF (mg) | CActual Al (mg/L) | |
| DC | 25.22 | 805.82 | 0.13 | 19.56 | 832.38 | 0.85 | 13.67 | 872.41 | 1.02 |
| APC (CCP = 1 min) | 23.43 | 830.98 | 0.22 | 16.15 | 912.17 | 0.78 | 11.58 | 946.70 | 1.11 |
| Current Type | CCP (min) | Energy Consumption (kWh/m3) | |||||
|---|---|---|---|---|---|---|---|
| j = 15 A/m2 | j = 30 A/m2 | j = 45 A/m2 | |||||
| SOT = 10 min | SOT = 90 min | SOT = 10 min | SOT = 90 min | SOT = 10 min | SOT = 90 min | ||
| DC | 0 | 3.38 × 10−3 | 3.46 × 10−3 | 7.10 × 10−3 | 7.34 × 10−3 | 11.99 × 10−3 | 12.54 × 10−3 |
| APC | 1 | 3.38 × 10−3 | 3.38 × 10−3 | 7.07 × 10−3 | 7.07 × 10−3 | 11.88 × 10−3 | 11.92 × 10−3 |
| 5 | 3.38 × 10−3 | 3.38 × 10−3 | 7.07 × 10−3 | 7.10 × 10−3 | 11.88 × 10−3 | 11.99 × 10−3 | |
| 10 | 3.38 × 10−3 | 3.42 × 10−3 | 7.10 × 10−3 | 7.17 × 10−3 | 11.99 × 10−3 | 12.21 × 10−3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Qiu, T.; Chen, F.; Sun, M.; Zhou, L.; Sun, J.; Du, Y. Enhancing the Performance of the Electrocoagulation−Filtration System Treating Mariculture Tailwaters by Using Alternating Pulse Current: Effects of Current Density and Current Conversion Period. Water 2022, 14, 1181. https://doi.org/10.3390/w14081181
Xu J, Qiu T, Chen F, Sun M, Zhou L, Sun J, Du Y. Enhancing the Performance of the Electrocoagulation−Filtration System Treating Mariculture Tailwaters by Using Alternating Pulse Current: Effects of Current Density and Current Conversion Period. Water. 2022; 14(8):1181. https://doi.org/10.3390/w14081181
Chicago/Turabian StyleXu, Jianping, Tianlong Qiu, Fudi Chen, Ming Sun, Li Zhou, Jianming Sun, and Yishuai Du. 2022. "Enhancing the Performance of the Electrocoagulation−Filtration System Treating Mariculture Tailwaters by Using Alternating Pulse Current: Effects of Current Density and Current Conversion Period" Water 14, no. 8: 1181. https://doi.org/10.3390/w14081181