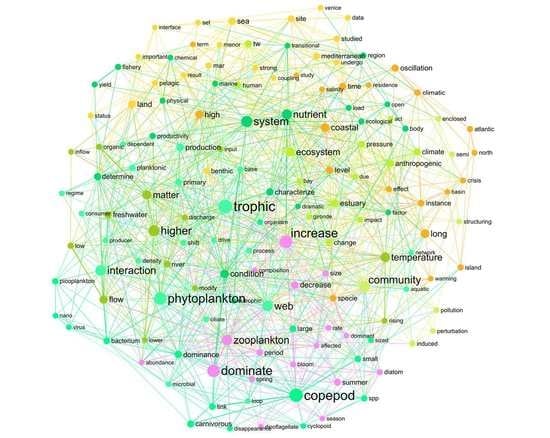
Plankton under Pressure: How Water Conditions Alter the Phytoplankton–Zooplankton Link in Coastal Lagoons
Abstract
:1. Introduction
2. Coastal Lagoons as Key Study Case for TWs
Impact of Hydrodynamic Changes on Lagoon Plankton
3. Rewiring of Planktonic Trophic Networks in Impacted TWs
4. Research Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voulvoulis, N.; Arpon, K.D.; Giakoumis, T. The EU Water Framework Directive: From great expectations to problems with implementation. Sci. Total Environ. 2017, 575, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliapietra, D.; Povilanskas, R.; Razinkovas-Baziukas, A.; Tamin, J. Emerald growth: A new framework concept for managing ecological quality and ecosystem services of transitional waters. Water 2020, 12, 894. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, H.; Lillebø, A.I.; Culhane, F.; Robinson, L.; Trauner, D.; Borgwardt, F.; Kummerlen, M.; Barbosa, A.; McDonald, H.; Funk, A.; et al. Linking biodiversity to ecosystem services supply: Patterns across aquatic ecosystems. Sci. Total Environ. 2019, 657, 517–534. [Google Scholar] [CrossRef] [PubMed]
- D’Alelio, D.; Russo, L.; Hay Mele, B.; Pomati, F. Intersecting Ecosystem Services Across the Aquatic Continuum: From Global Change Impacts to Local, and Biologically Driven, Synergies and Trade-Offs. Front. Ecol. Evol. 2021, 9, 1–9. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Pérez-Ruzafa, I.M.; Newton, A.; Marcos, C. Coastal Lagoons: Environmental Variability, Ecosystem Complexity, and Goods and Services Uniformity. In Coasts and Estuaries; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–276. ISBN 9780128140048. [Google Scholar]
- Ibánhez, J.S.P.; Arévalo, E.; Kelly, T.; Papaspyrou, S.; Rocha, C.; Nicolaidou, A. Unraveling the dispersion and environmental impact of anthropogenic discharges in transitional water ecosystems. Estuar. Coast. Shelf Sci. 2019, 216, 204–217. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ 13 C measurements as indicators of carbon flow in marine and freshwater ecosystems. In Stable Isotopes in Ecological Research; Springer: New York, NY, USA, 1989; Volume 47, pp. 196–229. [Google Scholar]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D. Complex seasonal patterns of primary producers at the land-sea interface. Ecol. Lett. 2008, 11, 1294–1303. [Google Scholar] [CrossRef]
- Rothenberger, M.B.; Swaffield, T.; Calomeni, A.J.; Cabrey, C.D. Multivariate analysis of water quality and plankton assemblages in an urban estuary. Estuaries Coasts 2014, 37, 695–711. [Google Scholar] [CrossRef]
- Araujo, A.V.; Dias, C.O.; Bonecker, S.L.C. Differences in the structure of copepod assemblages in four tropical estuaries: Importance of pollution and the estuary hydrodynamics. Mar. Pollut. Bull. 2017, 115, 412–420. [Google Scholar] [CrossRef]
- Hallett, C.S.; Hobday, A.J.; Tweedley, J.R.; Thompson, P.A.; McMahon, K.; Valesini, F.J. Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Reg. Environ. Chang. 2018, 18, 1357–1373. [Google Scholar] [CrossRef]
- Warwick, R.M.; Tweedley, J.R.; Potter, I.C. Microtidal estuaries warrant special management measures that recognise their critical vulnerability to pollution and climate change. Mar. Pollut. Bull. 2018, 135, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Critto, A.; Torresan, S.; Giubilato, E.; Santini, M.; Zirino, A.; Ouyang, W.; Marcomini, A. Modelling climate change impacts on nutrients and primary production in coastal waters. Sci. Total Environ. 2018, 628, 919–937. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ducrotoy, J.P.; Michael, E.; Cutts, N.D.; Franco, A.; Little, S.; Mazik, K.; Wilkinson, M. Temperate Estuaries: Their Ecology Under Future Environmental Changes. In Coasts and Estuaries; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–276. [Google Scholar] [CrossRef]
- Tarafdar, L.; Kim, J.Y.; Srichandan, S.; Mohapatra, M.; Muduli, P.R.; Kumar, A.; Mishra, D.R.; Rastogi, G. Responses of phytoplankton community structure and association to variability in environmental drivers in a tropical coastal lagoon. Sci. Total Environ. 2021, 783, 146873. [Google Scholar] [CrossRef]
- Haraguchi, L.; Carstensen, J.; Abreu, P.C.; Odebrecht, C. Long-term changes of the phytoplankton community and biomass in the subtropical shallow Patos Lagoon Estuary, Brazil. Estuar. Coast. Shelf Sci. 2015, 162, 76–87. [Google Scholar] [CrossRef]
- Jakimavičius, D.; Kriaučiūnienė, J.; Šarauskienė, D. Impact of climate change on the Curonian Lagoon water balance components, salinity and water temperature in the 21st century. Oceanologia 2018, 60, 378–389. [Google Scholar] [CrossRef]
- Morabito, G.; Mazzocchi, M.G.; Salmaso, N.; Zingone, A.; Bergami, C.; Flaim, G.; Accoroni, S.; Basset, A.; Bastianini, M.; Belmonte, G.; et al. Plankton dynamics across the freshwater, transitional and marine research sites of the LTER-Italy Network. Patterns, fluctuations, drivers. Sci. Total Environ. 2018, 627, 373–387. [Google Scholar] [CrossRef]
- Duck, R.W.; da Silva, J.F. Coastal lagoons and their evolution: A hydromorphological perspective. Estuar. Coast. Shelf Sci. 2012, 110, 2–14. [Google Scholar] [CrossRef]
- Martin, L.; Landim Dominguez, J.M. Geological History of Coastal Lagoons. In Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1994; Volume 60, pp. 41–68. [Google Scholar]
- Kennish, M.J.; Paerl, H.W.E. Coastal Lagoons: Critical Habitats of Environmental Change; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Barnes, R.S.K. Coastal Lagoons; Archive, C., Ed.; Cambridge Univ. Press: New York, NY, USA, 1980; Volume 1. [Google Scholar]
- Boyd, R.; Dalrymple, R.; Zaitlin, B.A. Classification of clastic coastal depositional environments. Sediment. Geol. 1992, 80, 139–150. [Google Scholar] [CrossRef]
- Tagliapietra, D.; Sigovini, M.; Ghirardini, A.V. A review of terms and definitions to categorise estuaries, lagoons and associated environments. Mar. Freshw. Res. 2009, 60, 497–509. [Google Scholar] [CrossRef]
- Colombo, G. Lagoons; Barnes, R., Ed.; John Wiley and Sons: New York, NY, USA, 1977. [Google Scholar]
- McGlathery, K.J.; Sundbäck, K.; Anderson, I.C. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar. Ecol. Prog. Ser. 2007, 348, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Sousa, A.I.; et al. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- Escudero, M.; Silva, R.; Mendoza, E. Beach erosion driven by natural and human activity at Isla del Carmen Barrier Island, Mexico. J. Coast. Res. 2014, 71, 62–74. [Google Scholar] [CrossRef]
- Madarasinghe, S.K.; Yapa, K.K.A.S.; Satyanarayana, B.; Udayakantha, P.M.P.; Kodikara, S.; Jayatissa, L.P. Inland Irrigation Project Causes Disappearance of Coastal Lagoon: The Trajectory of Kalametiya Lagoon, Sri Lanka from 1956 to 2016. Coast. Manag. 2020, 48, 188–209. [Google Scholar] [CrossRef]
- Carrasco, A.R.; Ferreira, O.; Roelvink, D. Coastal lagoons and rising sea level: A review. Earth-Sci. Rev. 2016, 154, 356–368. [Google Scholar] [CrossRef] [Green Version]
- Troiani, B.T.; Simms, A.R.; Dellapenna, T.; Piper, E.; Yokoyama, Y. The importance of sea-level and climate change, including changing wind energy, on the evolution of a coastal estuary: Copano Bay, Texas. Mar. Geol. 2011, 280, 1–12. [Google Scholar] [CrossRef]
- Toldo, J.; Dillenburg, S.R.; Correa, I.C.S.; Almeida, L.E.S.B. Holocene sedimentation in Lagoa dos Pantos Lagoon, Rio Grande do Sul, Brazil. J. Coast. Res. 2000, 16, 816–822. [Google Scholar]
- Sloss, C.R.; Jones, B.G.; McClennen, C.E.; de Carli, J.; Price, D.M. The geomorphological evolution of a wave-dominated barrier estuary: Burrill Lake, New South Wales, Australia. Sediment. Geol. 2006, 187, 229–249. [Google Scholar] [CrossRef] [Green Version]
- Ferla, M.; Cordella, M.; Michielli, L.; Rusconi, A. Long-term variations on sea level and tidal regime in the lagoon of Venice. Estuar. Coast. Shelf Sci. 2007, 75, 214–222. [Google Scholar] [CrossRef]
- Navrotskaya, S.E.; Chubarenko, B.V. Trends in the variation of the sea level in the lagoons of the Southeastern Baltic. Oceanology 2013, 53, 13–23. [Google Scholar] [CrossRef]
- Frihy, O.E.; El-Sayed, M.K. Vulnerability risk assessment and adaptation to climate change induced sea level rise along the Mediterranean coast of Egypt. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 1215–1237. [Google Scholar] [CrossRef]
- van de Plassche, O. Coastal Evolution: Late Quaternary Shoreline Morphodynamics; Cambridge University Press: Cambridge, England, 1994. [Google Scholar]
- Hibma, A.; Stive, M.J.F.; Wang, Z.B. Estuarine morphodynamics. Coast. Eng. 2004, 51, 765–778. [Google Scholar] [CrossRef]
- Madricardo, F.; Foglini, F.; Campiani, E.; Grande, V.; Catenacci, E.; Petrizzo, A.; Kruss, A.; Toso, C.; Trincardi, F. Assessing the human footprint on the sea-floor of coastal systems: The case of the Venice Lagoon, Italy. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ghezzo, M.; Guerzoni, S.; Cucco, A.; Umgiesser, G. Changes in Venice Lagoon dynamics due to construction of mobile barriers. Coast. Eng. 2010, 57, 694–708. [Google Scholar] [CrossRef] [Green Version]
- Basset, A.; Barbone, E.; Rosati, I.; Vignes, F.; Breber, P.; Specchiulli, A.; D′Adamo, R.; Renzi, M.; Focardi, S.; Ungaro, N.; et al. Resistance and resilience of ecosystem descriptors and properties to dystrophic events: A study case in a Mediterranean lagoon. Transit. Waters Bull. 2013, 7, 1–22. [Google Scholar] [CrossRef]
- Pitacco, V.; Reizopoulou, S.; Sfriso, A.; Sfriso, A.; Mistri, M.; Munari, C. The difficulty of disentangling natural from anthropogenic forcing factors makes the evaluation of ecological quality problematic: A case study from Adriatic lagoons. Mar. Environ. Res. 2019, 150, 104756. [Google Scholar] [CrossRef]
- Ramdani, M.; Elkhiati, N.; Flower, R.J.; Thompson, J.R.; Chouba, L.; Kraiem, M.M.; Ayache, F.; Ahmed, M.H. Environmental influences on the qualitative and quantitative composition of phytoplankton and zooplankton in North African coastal lagoons. Hydrobiologia 2009, 622, 113–131. [Google Scholar] [CrossRef]
- Silva, A.M.A.; Barbosa, J.E.; Medeiros, P.R.; Rocha, R.M.; Lucena-Filho, M.A.; Silva, D.F. Zooplankton (Cladocera and Rotifera) variations along a horizontal salinity gradient and during two seasons (dry and rainy) in a tropical inverse estuary (Northeast Brazil). Panam. J. Aquat. Sci. 2009, 4, 226–238. [Google Scholar]
- Hemraj, D.A.; Hossain, A.; Ye, Q.; Qin, J.G.; Leterme, S.C. Anthropogenic shift of planktonic food web structure in a coastal lagoon by freshwater flow regulation. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Paturej, E.; Gutkowska, A.; Koszałka, J.; Bowszys, M. Effect of physicochemical parameters on zooplankton in the brackish, coastal Vistula Lagoon. Oceanologia 2017, 59, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Gilabert, J. Seasonal plankton dynamics in a Mediterranean hypersaline coastal lagoon: The Mar Menor. J. Plankton Res. 2001, 23, 207–217. [Google Scholar] [CrossRef]
- Pérez Ruzafa, Á.; Marcos Diego, C.; Gilabert Cervera, F.J. The ecology of the Mar Menor coastal lagoon: A fast changing ecosystem under human pressure. In Coastal Lagoons. Ecosystem Processes and Modeling for Sustainable Use and Development; CRC Press: Boca Raton, FL, USA, 2005; pp. 392–422. [Google Scholar]
- Alongi, D.M. Coastal Ecosystem Processes; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Vizzini, S.; Mazzola, A. The fate of organic matter sources in coastal environments: A comparison of three Mediterranean lagoons. Hydrobiologia 2008, 611, 67–79. [Google Scholar] [CrossRef]
- Nowicki, B.; Nixon, S. Benthic community metabolism in a coastal lagoon ecosystem. Mar. Ecol. Prog. Ser. 1985, 22, 21–30. [Google Scholar] [CrossRef]
- Knowlton, N. Multiple “stable” states and the conservation of marine ecosystems. Prog. Oceanogr. 2004, 60, 387–396. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. Ecological evaluation of transitional and coastal waters: A marine benthic macrophytes-based model. Mediterr. Mar. Sci. 2001, 2, 45–66. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Viaroli, P.; Bartoli, M.; Giordani, G.; Naldi, M.; Orfanidis, S.Z.J. Community shifts, alternative stable states, biogeochemical controlsand feedbacks in eutrophic coastal lagoons: A brief overview. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, S105–S117. [Google Scholar] [CrossRef]
- Fong, P.; Kennison, R.L. Phase shifts, alternative stable states, and the status of Southern California lagoons. In Coastal Lagoons: Critical Habitats of Environmental Change; Michael, J., Kennish, H.W.P., Eds.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Odame Appiah, D.; Yankson, D. Anthropogenic Drivers of the Pressures on the Ramsar Site of Sakumo Lagoon in Ghana. Int. J. Technol. Manag. Res. 2020, 1, 48–56. [Google Scholar] [CrossRef]
- Koutsodendris, A.; Brauer, A.; Zacharias, I.; Putyrskaya, V.; Klemt, E.; Sangiorgi, F.; Pross, J. Ecosystem response to human- and climate-induced environmental stress on an anoxic coastal lagoon (Etoliko, Greece) since 1930 AD. J. Paleolimnol. 2015, 53, 255–270. [Google Scholar] [CrossRef]
- Pérez Ruzafa, A.; Munilla León, T. Pycnogoid ecology in the Mar Menor (Murcia, SW Mediterranean). Sci. Mar. 1992, 56, 21–28. [Google Scholar]
- Álvarez-Rogel, J.; Barberá, G.G.; Maxwell, B.; Guerrero-Brotons, M.; Díaz-García, C.; Martínez-Sánchez, J.J.; Sallent, A.; Martínez-Ródenas, J.; González-Alcaraz, M.N.; Jiménez-Cárceles, F.J.; et al. The case of Mar Menor eutrophication: State of the art and description of tested Nature-Based Solutions. Ecol. Eng. 2020, 158, 106086. [Google Scholar] [CrossRef]
- Lloret, J.; Marín, A.; Marín-Guirao, L. Is coastal lagoon eutrophication likely to be aggravated by global climate change? Estuar. Coast. Shelf Sci. 2008, 78, 403–412. [Google Scholar] [CrossRef]
- D’Alelio, D.; Libralato, S.; Wyatt, T.; Ribera D′Alcalà, M. Ecological-network models link diversity, structure and function in the plankton food-web. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alelio, D.; Hay Mele, B.; Libralato, S.; Ribera d′Alcalà, M.; Jordán, F. Rewiring and indirect effects underpin modularity reshuffling in a marine food web under environmental shifts. Ecol. Evol. 2019, 9, 11631–11646. [Google Scholar] [CrossRef]
- Maher, D.T.; Call, M.; Macklin, P.; Webb, J.R.; Santos, I.R. Hydrological Versus Biological Drivers of Nutrient and Carbon Dioxide Dynamics in a Coastal Lagoon. Estuaries Coasts 2019, 42, 1015–1031. [Google Scholar] [CrossRef]
- Rissik, D.; Shon, E.H.; Newell, B.; Baird, M.E.; Suthers, I.M. Plankton dynamics due to rainfall, eutrophication, dilution, grazing and assimilation in an urbanized coastal lagoon. Estuar. Coast. Shelf Sci. 2009, 84, 99–107. [Google Scholar] [CrossRef]
- Shangguan, Y. Response of plankton communities in coastal laggons to changes in nutrient quality and quantity: Case study of Florida Bay. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2016. [Google Scholar]
- Cutrim, M.V.J.; Ferreira, F.S.; Duarte dos Santos, A.K.; Cavalcanti, L.F.; de Oliveira Araújo, B.; de Azevedo-Cutrim, A.C.G.; Furtado, J.A.; Oliveira, A.L.L. Trophic state of an urban coastal lagoon (northern Brazil), seasonal variation of the phytoplankton community and environmental variables. Estuar. Coast. Shelf Sci. 2019, 216, 98–109. [Google Scholar] [CrossRef]
- Glibert, P.M.; Boyer, J.; Heil, C.; Madden, C.; Sturgis, B.W.C. Blooms in lagoons: Different from those of river-dominated estuaries. In Coastal Lagoons: Critical Habitats of Environmental Change; Kennish, M.J., Paerl, H., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 91–114. [Google Scholar]
- Glibert, P.M.; Hinkle, D.C.; Sturgis, B.; Jesien, R.V. Eutrophication of a Maryland/Virginia Coastal Lagoon: A Tipping Point, Ecosystem Changes, and Potential Causes. Estuaries Coasts 2014, 37, 128–146. [Google Scholar] [CrossRef] [Green Version]
- Malone, T.C.; Conley, D.J.; Fisher, T.R.; Glibert, P.M.; Harding, L.W.; Sellner, K.G. Scales of Nutrient-Limited Phytoplankton Productivity in Chesapeake Bay. Estuaries 1996, 19, 371–385. [Google Scholar] [CrossRef]
- Legendre, L.; Rassoulzadegan, F. Plankton and nutrient dynamics in marine waters. Ophelia 1995, 41, 153–172. [Google Scholar] [CrossRef]
- Deininger, A.; Faithfull, C.L.; Lange, K.; Bayer, T.; Vidussi, F.; Liess, A. Simulated terrestrial runoff triggered a phytoplankton succession and changed seston stoichiometry in coastal lagoon mesocosms. Mar. Environ. Res. 2016, 119, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, J.; Brugel, S.; Rowe, O.; Lefébure, R.; Brutemark, A.; Andersson, A. Response of Coastal Phytoplankton to High Inflows of Terrestrial Matter. Front. Mar. Sci. 2020, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Donázar-Aramendía, I.; Sánchez-Moyano, J.E.; García-Asencio, I.; Miró, J.M.; Megina, C.; García-Gómez, J.C. Human pressures on two estuaries of the Iberian Peninsula are reflected in food web structure. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Najjar, R.G.; Pyke, C.R.; Adams, M.B.; Breitburg, D.; Hershner, C.; Kemp, M.; Howarth, R.; Mulholland, M.R.; Paolisso, M.; Secor, D.; et al. Potential climate-change impacts on the Chesapeake Bay. Estuar. Coast. Shelf Sci. 2010, 86, 1–20. [Google Scholar] [CrossRef]
- Johannes, R.E.; Hearn, C.J. The effect of submarine groundwater discharge on nutrient and salinity regimes in a coastal lagoon off Perth, Western Australia. Estuar. Coast. Shelf Sci. 1985, 21, 789–800. [Google Scholar] [CrossRef]
- Cravo, A.; Barbosa, A.B.; Correia, C.; Matos, A.; Caetano, S.; Lima, M.J.; Jacob, J. Unravelling the effects of treated wastewater discharges on the water quality in a coastal lagoon system (Ria Formosa, South Portugal): Relevance of hydrodynamic conditions. Mar. Pollut. Bull. 2022, 174, 113296. [Google Scholar] [CrossRef]
- Meddeb, M.; Grami, B.; Chaalali, A.; Haraldsson, M.; Niquil, N.; Pringault, O.; Sakka Hlaili, A. Plankton food-web functioning in anthropogenically impacted coastal waters (SW Mediterranean Sea): An ecological network analysis. Prog. Oceanogr. 2018, 162, 66–82. [Google Scholar] [CrossRef] [Green Version]
- Stec, K.F.; Caputi, L.; Buttigieg, P.L.; D’Alelio, D.; Ibarbalz, F.M.; Sullivan, M.B.; Chaffron, S.; Bowler, C.; Ribera d′Alcalà, M.; Iudicone, D. Modelling plankton ecosystems in the meta-omics era. Are we ready? Mar. Genom. 2017, 32, 1–17. [Google Scholar] [CrossRef]
- D’Alelio, D.; Eveillard, D.; Coles, V.J.; Caputi, L.; Ribera d′Alcalà, M.; Iudicone, D. Modelling the complexity of plankton communities exploiting omics potential: From present challenges to an integrative pipeline. Curr. Opin. Syst. Biol. 2019, 13, 68–74. [Google Scholar] [CrossRef]
- Karsenti, E.; Acinas, S.G.; Bork, P.; Bowler, C.; De Vargas, C.; Raes, J. Tara Oceans Consortium A Holistic Approach to Marine Eco-Systems Biology. PLoS Biol. 2011, 9, e1001177. [Google Scholar] [CrossRef]
- Ghai, R.; Hernandez, C.M.; Picazo, A.; Mizuno, C.M.; Ininbergs, K.; Díez, B.; Valas, R.; Dupont, C.L.; McMahon, K.D.; Camacho, A.; et al. Metagenomes of mediterranean coastal lagoons. Sci. Rep. 2012, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, L.J.; Turner, J.W. Shotgun metagenomics reveals the benthic microbial community response to plastic and bioplastic in a coastal marine environment. Front. Microbiol. 2019, 10, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, A.B.; Ghai, R.; Martin-Cuadrado, A.B.; Sánchez-Porro, C.; Rodriguez-Valera, F.; Ventosa, A. Prokaryotic taxonomic and metabolic diversity of an intermediate salinity hypersaline habitat assessed by metagenomics. FEMS Microbiol. Ecol. 2014, 88, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, M.; Pauletto, M.; Boffo, L.; Carrer, C.; Sorrentino, F.; Ferrari, G.; Pavan, L.; Patarnello, T.; Bargelloni, L. Transcriptomic resources for environmental risk assessment: A case study in the Venice lagoon. Environ. Pollut. 2015, 197, 90–98. [Google Scholar] [CrossRef]
- Acri, F.; Aubry, F.B.; Berton, A.; Bianchi, F.; Boldrin, A.; Camatti, E.; Comaschi, A.; Rabitti, S.; Socal, G. Plankton communities and nutrients in the Venice Lagoon. Comparison between current and old data. J. Mar. Syst. 2004, 51, 321–329. [Google Scholar] [CrossRef]
- Pulina, S.; Suikkanen, S.; Padedda, B.M.; Brutemark, A.; Grubisic, L.M.; Satta, C.T.; Caddeo, T.; Farina, P.; Lugliè, A. Responses of a Mediterranean coastal lagoon plankton community to experimental warming. Mar. Biol. 2020, 167, 1–14. [Google Scholar] [CrossRef]
- Courboulès, J.; Vidussi, F.; Soulié, T.; Mas, S.; Pecqueur, D.; Mostajir, B. Effects of experimental warming on small phytoplankton, bacteria and viruses in autumn in the Mediterranean coastal Thau Lagoon. Aquat. Ecol. 2021, 55, 647–666. [Google Scholar] [CrossRef]
- Sultana, R.; Casareto, B.E.; Sohrin, R.; Suzuki, T.; Alam, M.S.; Fujimura, H.; Suzuki, Y. Response of subtropical coastal sediment systems of Okinawa, Japan, to experimental warming and high pCO2. Front. Mar. Sci. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Alsterberg, C.; Sundbäck, K.; Hulth, S. Functioning of a Shallow-Water Sediment System during Experimental Warming and Nutrient Enrichment. PLoS ONE 2012, 7, e51503. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alelio, D.; Russo, L.; Del Gaizo, G.; Caputi, L. Plankton under Pressure: How Water Conditions Alter the Phytoplankton–Zooplankton Link in Coastal Lagoons. Water 2022, 14, 974. https://doi.org/10.3390/w14060974
D’Alelio D, Russo L, Del Gaizo G, Caputi L. Plankton under Pressure: How Water Conditions Alter the Phytoplankton–Zooplankton Link in Coastal Lagoons. Water. 2022; 14(6):974. https://doi.org/10.3390/w14060974
Chicago/Turabian StyleD’Alelio, Domenico, Luca Russo, Gabriele Del Gaizo, and Luigi Caputi. 2022. "Plankton under Pressure: How Water Conditions Alter the Phytoplankton–Zooplankton Link in Coastal Lagoons" Water 14, no. 6: 974. https://doi.org/10.3390/w14060974