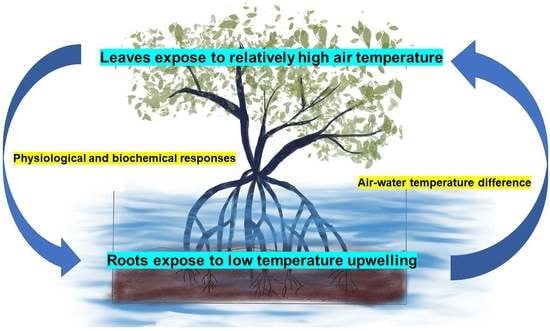
Physiological and Biochemical Responses of Kandelia obovata to Upwelling Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seedling Collection and Treatment Conditions
2.2. Determination Method
2.2.1. Determination of Chlorophyll Content and Fluorescence Parameters
2.2.2. Determination of Soluble Sugar and Proline
2.2.3. Extraction and Assay of H2O2, MDA, SOD, POD, and CAT
2.3. Statistical Analyses
3. Results
3.1. Changes in Photosynthetic Pigment and Fluorescence Parameters
3.2. Changes in H2O2 Production and MDA Content
3.3. Changes in Proline and Soluble Sugar Content
3.4. Changes in SOD, POD, and CAT Activity
3.5. Principal Component Analysis (PCA)
4. Discussion
4.1. Response of the Photosynthetic System of K. obovata Seedlings to Upwelling
4.2. Membrane Damage and Oxidative Stress of K. obovata Seedlings under Upwelling
4.3. Response of Antioxidant Mechanisms of K. obovata Seedlings to Upwelling
4.4. Relationship between Upwelling Treatment and K. obovata Seedling Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tatongjai, S.; Kraichak, E.; Kermanee, P. Comparative anatomy and salt management of Sonneratia caseolaris (L.) Engl. (Lythraceae) grown in saltwater and freshwater. PeerJ 2021, 9, e10962. [Google Scholar] [CrossRef] [PubMed]
- Himes-Cornell, A.; Grose, S.O.; Pendleton, L. Mangrove ecosystem service values and methodological approaches to valuation: Where do we stand? Front. Mar. Sci. 2018, 5, 376. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Gu, J.-D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegradat. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Delfan, N.; Shojaei, M.G.; Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Estuarine Coast. Shelf Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef] [Green Version]
- Kathiresan, K.; Rajendran, N. Coastal mangrove forests mitigated tsunami. Estuarine Coast. Shelf Sci. 2005, 65, 601–606. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.-S.; Sun, C.-C.; Wang, Y.-T.; Peng, Y.-L.; Cheng, H. Effects of pyrene on antioxidant systems and lipid peroxidation level in mangrove plants, Bruguiera gymnorrhiza. Ecotoxicology 2012, 21, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Sun, X.; Xu, Y.; Zhang, Q.; Li, X. Accumulation and tolerance of mangroves to heavy metals: A review. Curr. Pollut. Rep. 2017, 3, 302–317. [Google Scholar] [CrossRef]
- Anneboina, L.R.; Kumar, K.K. Economic analysis of mangrove and marine fishery linkages in India. Ecosyst. Serv. 2017, 24, 114–123. [Google Scholar] [CrossRef]
- Walters, B.B.; Rönnbäck, P.; Kovacs, J.M.; Crona, B.; Hussain, S.A.; Badola, R.; Primavera, J.H.; Barbier, E.; Dahdouh-Guebas, F. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat. Bot. 2008, 89, 220–236. [Google Scholar] [CrossRef] [Green Version]
- Padhy, S.R.; Bhattacharyya, P.; Dash, P.K.; Reddy, C.S.; Chakraborty, A.; Pathak, H. Seasonal fluctuation in three mode of greenhouse gases emission in relation to soil labile carbon pools in degraded mangrove, Sundarban, India. Sci. Total Environ. 2020, 705, 135909. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Machado, W.; Sanders, C.J. Anthropogenic and environmental influences on nutrient accumulation in mangrove sediments. Mar. Pollut. Bull. 2021, 165, 112174. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.B.; Fourqurean, J.; Kauffman, J.B.; Marbà, N.; et al. Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodroffe, C.; Rogers, K.; McKee, K.; Lovelock, C.; Mendelssohn, I.; Saintilan, N. Mangrove Sedimentation and Response to Relative Sea-Level Rise. Annu. Rev. Mar. Sci. 2016, 8, 243–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Han, J.; Cheung, S.G.; Chong, R.K.Y.; Lo, C.-M.; Lee, F.W.-F.; Xu, S.J.-L.; Yang, Y.; Tam, N.F.-Y.; Zhou, H.-C. How mangrove plants affect microplastic distribution in sediments of coastal wetlands: Case study in Shenzhen Bay, South China. Sci. Total Environ. 2020, 767, 144695. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D. Marine debris: A proximate threat to marine sustainability in Bootless Bay, Papua New Guinea. Mar. Pollut. Bull. 2012, 64, 1880–1883. [Google Scholar] [CrossRef] [PubMed]
- Leoville, A.; Lagarde, R.; Grondin, H.; Faivre, L.; Rasoanirina, E.; Teichert, N. Influence of environmental conditions on the distribution of burrows of the mud crab, Scylla serrata, in a fringing mangrove ecosystem. Reg. Stud. Mar. Sci. 2021, 43, 101684. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yang, S.; Li, X.; Fang, L.; Zhong, C.; Duke, N.C.; Zhou, R.; Shi, S. Effects of Pleistocene sea-level fluctuations on mangrove population dynamics: A lesson from Sonneratia alba. BMC Evol. Biol. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Q.; Wang, Z.; Tao, J.; Kimura, M.K.; Liu, H.; Hogetsu, T.; Lian, C. Ocean currents drove genetic structure of seven dominant mangrove species along the coastlines of Southern China. Front. Genet. 2021, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Singh, M.; Wang, J.; Xiao, L.; Guan, D. Effects of marine pollution, climate, and tidal range on biomass and sediment organic carbon in Chinese mangrove forests. Catena 2021, 202, 105270. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Li, Q.Q.; Zhang, Y.; Yang, S.; Osland, M.J.; Huang, J.; Peng, C. Mangrove species’ responses to winter air temperature extremes in China. Ecosphere 2017, 8, e01865. [Google Scholar] [CrossRef]
- Barnuevo, A.; Asaeda, T. Integrating the ecophysiology and biochemical stress indicators into the paradigm of mangrove ecology and a rehabilitation blueprint. PLoS ONE 2018, 13, e0202227. [Google Scholar] [CrossRef] [Green Version]
- Du, H.Y.; Zhao, J.; Shi, Y.F.; Che, Y.J. The succession of potential vegetation in China and its sensitivity under climate change. Chin. J. Ecol. 2018, 37, 1459–1466. [Google Scholar] [CrossRef]
- Cheng, H.; Inyang, A.; Li, C.-D.; Fei, J.; Zhou, Y.-W.; Wang, Y.-S. Salt tolerance and exclusion in the mangrove plant Avicennia marina in relation to root apoplastic barriers. Ecotoxicology 2020, 29, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Shen, Z.-J.; Luo, M.-R.; Liu, Y.-L.; Wei, M.-Y.; Wang, W.-H.; Qin, Y.-Y.; Gao, C.-H.; Li, K.-K.; et al. Physiological and proteomic responses of mangrove plant Avicennia marina seedlings to simulated periodical inundation. Plant Soil 2020, 450, 231–254. [Google Scholar] [CrossRef]
- Lu, X.; Huang, C.; Chen, F.; Zhang, S.; Lao, Q.; Chen, C.; Wu, J.; Jin, G.; Zhu, Q. Carbon and nitrogen isotopic compositions of particulate organic matter in the upwelling zone off the east coast of Hainan Island, China. Mar. Pollut. Bull. 2021, 167, 112349. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, N.; Bai, M.; Li, J.; Wang, G. Composition change and decreased diversity of microbial eukaryotes in the coastal upwelling waters of South China Sea. Sci. Total Environ. 2021, 795, 148892. [Google Scholar] [CrossRef] [PubMed]
- Krek, A.; Krek, E.; Danchenkov, A.; Krechik, V.; Kapustina, M. The role of upwellings in the coastal ecosystem of the Southeastern Baltic Sea. Reg. Stud. Mar. Sci. 2021, 44, 101707. [Google Scholar] [CrossRef]
- Collin, R.; Ochoa, I. Influence of seasonal environmental variation on the reproduction of four tropical marine gastropods. Mar. Ecol. Prog. Ser. 2016, 555, 125–139. [Google Scholar] [CrossRef]
- Risheng Wu, L.L. Summarization of study on upwelling system in the South China Sea. J. Oceanogr. Taiwan Strait 2003, 22, 8. [Google Scholar]
- Jing, Z.-Y.; Qi, Y.-Q.; Hua, Z.-L.; Zhang, H. Numerical study on the summer upwelling system in the northern continental shelf of the South China Sea. Cont. Shelf Res. 2009, 29, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Huang, Z.; Hu, J. Using TPI to map spatial and temporal variations of significant coastal upwelling in the Northern South China Sea. Remote Sens. 2021, 13, 1065. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, Y.-S. Modeling the ecosystem response to summer coastal upwelling in the northern South China Sea. Oceanologia 2018, 60, 32–51. [Google Scholar] [CrossRef]
- Chen, C.-C.; Shiah, F.-K.; Gong, G.-C.; Chen, T.-Y. Impact of upwelling on phytoplankton blooms and hypoxia along the Chinese coast in the East China Sea. Mar. Pollut. Bull. 2021, 167, 112288. [Google Scholar] [CrossRef]
- Pan, D.; Wang, L.; Tan, F.; Lu, S.; Lv, X.; Zaynab, M.; Cheng, C.-L.; Abubakar, Y.S.; Chen, S.; Chen, W. Phosphoproteomics unveils stable energy supply as key to flooding tolerance in Kandelia candel. J. Proteom. 2018, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, P. Mangrove Ecosystem in China; Science Press: Beijing, China, 1997. [Google Scholar]
- Lang, T.; Wei, P.; Chen, X.; Fu, Y.; Tam, N.; Hu, Z.; Chen, Z.; Li, F.; Zhou, H. Microcosm study on allelopathic effects of leaf litter leachates and purified condensed tannins from Kandelia obovata on germination and growth of Aegiceras corniculatum. Forests 2021, 12, 1000. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Shen, Z.-J.; Simon, M.; Li, H.; Ma, D.-N.; Zhu, X.-Y.; Zheng, H.-L. Comparative proteomic analysis reveals the regulatory effects of h2s on salt tolerance of mangrove plant Kandelia obovata. Int. J. Mol. Sci. 2019, 21, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.-L.; Wang, Y.-S.; Fei, J.; Sun, C.-C. Isolation and expression analysis of two novel C-repeat binding factor (CBF) genes involved in plant growth and abiotic stress response in mangrove Kandelia obovata. Ecotoxicology 2020, 29, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Jian-Jun Hao, Z.-L.K.; Yang, Y. Experimental Rechnique of Plant Physiology; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Gan, T.; Zhao, N.; Yin, G.; Chen, M.; Wang, X.; Liu, J.; Liu, W. Optimal chlorophyll fluorescence parameter selection for rapid and sensitive detection of lead toxicity to marine microalgae Nitzschia closterium based on chlorophyll fluorescence technology. J. Photochem. Photobiol. B Biol. 2019, 197, 111551. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.-q.; Chai, J.; Wang, M.; Jia, X.; Guo, Z.; Sun, L.; Nie, X. Effect of barley yellow dwarf virus infection on photosynthesis and chlorophyll fluorescence parameters of oat. ACTA Agrestia Sin. 2020, 28, 923–931. [Google Scholar] [CrossRef]
- Tao, L.; Ren, H.; Ren, J. Assessment of cultured media for desert moss crust by physiological responses. J. Basic Microbiol. 2021, 61, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Guizhu, C. Physiological adaptability of three mangrove species to salt stress. Acta Ecol. Sin. 2007, 27, 2208–2214. [Google Scholar] [CrossRef]
- Afefe, A.A.; Khedr, A.-H.A.; Abbas, M.S.; Soliman, A.S. Responses and Tolerance Mechanisms of Mangrove Trees to the Ambient Salinity along the Egyptian Red Sea Coast. Limnol. Rev. 2021, 21, 3–13. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Zhang, T.; Zhang, Q.; Zhao, J. iTRAQ-Based Quantitative Proteomic Analysis Reveals Toxicity Mechanisms in Chlamys farreri Exposed to Okadaic Acid. Front. Mar. Sci. 2021, 8, 792050. [Google Scholar] [CrossRef]
- Ru, Q.M.; Xiao, Q.; Lin, P.; Pei, Z.M.; Zheng, H.L. Short- and long-term effects of NaCl on physiological and biochemical characteristics in leaves of a true mangrove, Kandelia candel. Russ. J. Plant Physiol. 2009, 56, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Lu, H.; Wang, Y.; Hong, H.; Wang, Q.; Liu, J.; Yan, C. Uptake, biotransformation and physiological response of TBBPA in mangrove plants after hydroponics exposure. Mar. Pollut. Bull. 2020, 151, 110832. [Google Scholar] [CrossRef]
- Li, H.-X.; Xiao, Y.; Cao, L.-L.; Yan, X.; Li, C.; Shi, H.-Y.; Wang, J.-W.; Ye, Y.-H. Cerebroside C Increases Tolerance to Chilling Injury and Alters Lipid Composition in Wheat Roots. PLoS ONE 2013, 8, e73380. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yu, K.; He, T.; Li, F.; Zhang, D.; Liu, J. The low temperature induced physiological responses of Avena nuda L.; a cold-tolerant plant species. Sci. World J. 2013, 2013, 658793. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; An, B.; Cao, D.; Xu, R.; Wang, S.; Zhang, Z.; Liu, X.; Sun, X. Improving photosynthetic capacity, alleviating photosynthetic inhibition and oxidative stress under low temperature stress with exogenous hydrogen sulfide in blueberry seedlings. Front. Plant Sci. 2020, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Xue, C.; Chen, H.; He, C.; Wang, Q. Low-Temperature Adaptation of the Snow Alga Chlamydomonas nivalis Is Associated With the Photosynthetic System Regulatory Process. Front. Microbiol. 2020, 11, 1233. [Google Scholar] [CrossRef]
- Wunderlich, A.; Pinheiro, M.A.A. Mangrove habitat partitioning by Ucides cordatus (Ucididae): Effects of the degree of tidal flooding and tree-species composition during its life cycle. Helgol. Mar. Res. 2012, 67, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Lin, K.-H.; Jiang, J.-Y.; Wang, C.-W.; Chen, C.-I.; Huang, M.-Y.; Weng, J.-H. Comparisons between yellow and green leaves of sweet potato cultivars in chlorophyll fluorescence during various temperature regimes under high light intensities. Sci. Hortic. 2021, 288, 110335. [Google Scholar] [CrossRef]
- Li, X.-G.; Meng, Q.-W.; Jiang, G.-Q.; Zou, Q. the susceptibility of cucumber and sweet pepper to chilling under low irradiance is related to energy dissipation and water-water cycle. Photosynthetica 2003, 41, 259–265. [Google Scholar] [CrossRef]
- Popov, V.N.; Antipina, O.V.; Selivanov, A.A.; Rakhmankulova, Z.F.; Deryabin, A. Functional activity of the photosynthetic apparatus in tobacco and arabidopsis plants exposed to chilling temperatures. Russ. J. Plant Physiol. 2019, 66, 102–109. [Google Scholar] [CrossRef]
- Farzana, S.; Chen, J.; Pan, Y.; Wong, Y.-S.; Tam, N.F.Y. Antioxidative response of Kandelia obovata, a true mangrove species, to polybrominated diphenyl ethers (BDE-99 and BDE-209) during germination and early growth. Mar. Pollut. Bull. 2016, 124, 1063–1070. [Google Scholar] [CrossRef]
- Kamran, R.V.; Toorchi, M.; Moghadam, M.; Mohammadi, H. The effect of cold stress on H2O2 and MDA contents in barely genotypes. J. Biodivers. Enviromental Sci. 2015, 7, 66–75. [Google Scholar]
- Xu, C.; Wang, M.; Yang, Z.; Zheng, Q. Low temperature and low irradiation induced irreversible damage of strawberry seedlings. Photosynthetica 2020, 58, 156–164. [Google Scholar] [CrossRef]
- Sun, W.H.; Duan, M.; Li, F.; Shu, D.F.; Yang, S.; Meng, Q.W. Overexpression of tomato tAPX gene in tobacco improves tolerance to high or low temperature stress. Biol. Plant. 2010, 54, 614–620. [Google Scholar] [CrossRef]
- Janků, M.; Luhová, L.; Petřivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Amari, T.; Abdelly, C. Biochemical responses of Digitaria commutata and Cenchrus ciliaris to water stress: Antioxidative reactions, proline and soluble sugars accumulation. Bioagro 2021, 33, 171–180. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Jia, F.-F.; Zhang, X.-M.; Qiao, Y.-X.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Temperature effects on the reactive oxygen species formation and antioxidant defence in roots of two cucurbit species with contrasting root zone temperature optima. Acta Physiol. Plant. 2011, 34, 713–720. [Google Scholar] [CrossRef]
- Liang, F.; Pan, Y.J.; Deng, X.; Wu, Y.S.; Liang, Z.R.; Zhao, S.H.; Tian, X.H. Responses of Barringtonia racemosa to Tidal Flooding. Fujian J. Agric. Sci. 2020, 35, 1346–1356. [Google Scholar] [CrossRef]
- de Oliveira, M.M.T.; Lu, S.; Zurgil, U.; Raveh, E.; Tel-Zur, N. Grafting in Hylocereus (Cactaceae) as a tool for strengthening tolerance to high temperature stress. Plant Physiol. Biochem. 2021, 160, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhou, J.; Di, B.; Liu, Y.; Zhang, G.; Yang, X. Using electrical impedance tomography for rapid determination of starch and soluble sugar contents in Rosa hybrida. Sci. Rep. 2021, 11, 2871. [Google Scholar] [CrossRef] [PubMed]
- Mohammadrezakhani, S.; Rezanejad, F.; Hajilou, J. Effect of putrescine and proline on proflies of GABA, antioxidant activities in leaves of three Citrus species in response to low temperature stress. J. Plant Biochem. Biotechnol. 2021, 30, 545–553. [Google Scholar] [CrossRef]
- Bao, G.; Tang, W.; An, Q.; Liu, Y.; Tian, J.; Zhao, N.; Zhu, S. Physiological effects of the combined stresses of freezing-thawing, acid precipitation and deicing salt on alfalfa seedlings. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef]
- Kalisz, A.; Gil, J.; Kunicki, E.; Sękara, A.; Sałata, A.; Caruso, G. Different temperature regimes influenced the quality of broccoli seedlings, which caused a change in the chemical composition of mature heads. Agronomy 2021, 11, 1806. [Google Scholar] [CrossRef]
- Xu, C. Changes of three osmotic regulatory metabolites contents in leaves of bamboo under low temperature stress. J. Henan Agric. Sci. 2011, 40, 127–130. [Google Scholar] [CrossRef]
- Ok, J.; Kim, S.H.; Ma, K.B.; Kim, D.; Jeong, H.S.; Shin, H. Seaonal fluctuation of freezing tolerance and soluble sugar content in three sweet persimmon cultivars. Hortic. Sci. Technol. 2020, 39, 305–313. [Google Scholar] [CrossRef]
- Das, L.; Patel, R.; Salvi, H.; Kamboj, R. Assessment of natural regeneration of mangrove with reference to edaphic factors and water in Southern Gulf of Kachchh, Gujarat, India. Heliyon 2019, 5, e02250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Zhang, Q.; Zheng, X.; Zhai, J.; Peng, C. Comparison of leaves and stems of Paederia scandens (Lour.) Merr. in tolerance to low temperature. Photosynthetica 2020, 58, 846–852. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, J.Z. Effects of Low Temperature Stress on Resistance Indices of Sympodial Bamboo Seedlings. Adv. Mater. Res. 2013, 726–731, 4241–4247. [Google Scholar] [CrossRef]
- Shen, Z.-J.; Qin, Y.-Y.; Luo, M.-R.; Li, Z.; Ma, D.-N.; Wang, W.-H.; Zheng, H.-L. Proteome analysis reveals a systematic response of cold-acclimated seedlings of an exotic mangrove plant Sonneratia apetala to chilling stress. J. Proteom. 2021, 248, 104349. [Google Scholar] [CrossRef]
- Miyama, M.; Tada, Y. Transcriptional and physiological study of the response of Burma mangrove (Bruguiera gymnorhiza) to salt and osmotic stress. Plant Mol. Biol. 2008, 68, 119–129. [Google Scholar] [CrossRef]





| Control | Treated | |||
|---|---|---|---|---|
| 25 °C | 13 °C | 5 °C | p-Value | |
| Photosynthetic pigments, mg/L | ||||
| Chlorophyll a | 7.458 ± 1.045 | 8.358 ± 1.769 | 7.611 ± 1.817 | 0.767 |
| Chlorophyll b | 2.902 ± 0.613 | 3.179 ± 0.584 | 2.856 ± 0.545 | 0.772 |
| Photosynthetic fluorescence parameters | ||||
| Y(Ⅱ) | 0.387 ± 0.047 | 0.348 ± 0.041 | 0.324 ± 0.021 | 0.197 |
| ETR | 70.233 ± 8.451 | 63 ± 7.375 | 58.633 ± 3.88 | 0.193 |
| qP | 0.542 ± 0.061 | 0.542 ± 0.066 | 0.504 ± 0.054 | 0.692 |
| qN | 0.459 ± 0.02 b | 0.659 ± 0.017 a | 0.678 ± 0.041 a | 0.000 |
| Fv/Fm | 0.833 ± 0.003 | 0.842 ± 0.003 | 0.845 ± 0.011 | 0.120 |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalue | 6.700 | 2.541 | 1.875 |
| Proportion % | 47.86 | 18.15 | 13.39 |
| Cumulative % | 47.86 | 66.01 | 79.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Y.; Dong, J.; Wu, M. Physiological and Biochemical Responses of Kandelia obovata to Upwelling Stress. Water 2022, 14, 899. https://doi.org/10.3390/w14060899
Li X, Wang Y, Dong J, Wu M. Physiological and Biochemical Responses of Kandelia obovata to Upwelling Stress. Water. 2022; 14(6):899. https://doi.org/10.3390/w14060899
Chicago/Turabian StyleLi, Xiaomei, Youshao Wang, Junde Dong, and Meilin Wu. 2022. "Physiological and Biochemical Responses of Kandelia obovata to Upwelling Stress" Water 14, no. 6: 899. https://doi.org/10.3390/w14060899