Purification of Micro-Polluted Lake Water by Biofortification of Vertical Subsurface Flow Constructed Wetlands in Low-Temperature Season
Abstract
:1. Introduction
2. Materials and Methods
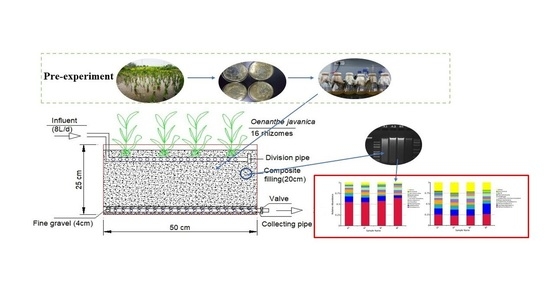
2.1. Experimental Wetland System
2.1.1. Design and Operation of Wetland
2.1.2. Plant Culture and Operation
2.1.3. Preparation of a Low-Temperature Resistant Microbial Agents
2.2. Sample Collection and Analytical Methods
2.2.1. Water Sampling and Analysis
2.2.2. Plant Monitoring and Analyses
2.2.3. Soil Microbial DNA Extraction and High-Throughput Sequencing Analysis
2.3. Data Analysis
3. Results and Discussion
3.1. Plant Growth Characteristics and Biomass Changes
3.2. Wastewater Purification Performance of Wetland System
3.3. Microbial Community Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, R.; Zhu, H.; Shutes, B.; Yan, B. Treatment of microcystin (MC-LR) and nutrients in eutrophic water by constructed wet lands: Performance and microbial community. Chemosphere 2021, 263, 128139. [Google Scholar] [CrossRef]
- Deng, S.; Chen, J.; Chang, J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits. J. Clean. Prod. 2021, 293, 126156. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Puigagut, J.; Caselles-Osorio, A.; Vaello, N.; García, J. Fractionation, Biodegradability and Particle-Size Distribution of Organic Matter in Horizontal Subsurface-Flow Constructed Wetlands; Springer: Dordrecht, The Netherlands, 2008; Volume 8, pp. 289–297. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.; Ma, N.; Wang, W.; Ma, C.; Zhang, R. Cadmium removal capability and growth characteristics of Iris sibirica in subsurface vertical flow constructed wetlands. Ecol. Eng. 2015, 84, 443–450. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.; Liu, Y.; Li, X.; Fan, X.; Zhou, Z.; Liu, C.; Yin, W. Enhanced simultaneous organics and nutrients removal in tidal flow constructed wetland using activated alumina as substrate treating domestic wastewater. Bioresour. Technol. 2019, 280, 441–446. [Google Scholar] [CrossRef]
- Chand, N.; Suthar, S.; Kumar, K.; Tyagi, V.K. Enhanced removal of nutrients and coliforms from domestic wastewater in cattle dung biochar-packed Colocasia esculenta-based vertical subsurface flow constructed wetland. J. Water Process. Eng. 2021, 41, 101994. [Google Scholar] [CrossRef]
- Baptista, J.D.C.; Davenport, R.J.; Donnelly, T.; Curtis, T.P. The microbial diversity of laboratory-scale wetlands appears to be randomly assembled. Water Res. 2008, 42, 3182–3190. [Google Scholar] [CrossRef]
- Si, Z.; Song, X.; Wang, Y.; Cao, X.; Zhao, Y.; Wang, B.; Chen, Y.; Arefe, A. Intensified heterotrophic denitrification in constructed wetlands using four solid carbon sources: Denitrification efficiency and bacterial community structure. Bioresour. Technol. 2018, 267, 416–425. [Google Scholar] [CrossRef]
- Zheng, Y.; Dzakpasu, M.; Wang, X.; Zhang, L.; Ngo, H.H.; Guo, W.; Zhao, Y. Molecular characterization of long-term impacts of macrophytes harvest management in constructed wetlands. Bioresour. Technol. 2018, 268, 514–522. [Google Scholar] [CrossRef]
- Tu, Y.; Li, H.; Dong, K.; Li, Q.; Jiang, L. Purification Efficiency under the Combined Function of 4 Plants on Domestic Sewage. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 62038. [Google Scholar] [CrossRef]
- Piñeyro, M.; Chalar, G.; Quintans, F. Constructed wetland scale model: Organic matter and nutrients removal from the effluent of a fish processing plant. Int. J. Environ. Sci. Technol. 2019, 16, 4181–4192. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Shao, Y.; Li, Z. How to increase microbial degradation in constructed wetlands: Influencing factors and improvement measures. Bioresour. Technol. 2014, 157, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dutta, V. Constructed wetland microcosms as sustainable technology for domestic wastewater treatment: An over view. Environ. Sci. Pollut. Res. 2019, 26, 11662–11673. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Yin, X. Improving low-temperature performance of surface flow constructed wetlands using Potamogeton crispus L. plant. Bioresour. Technol. 2016, 218, 1257–1260. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Li, P.; Zhang, J.; Xie, H.; Zhang, B. Nutrient removal in constructed microcosm wetlands for treating polluted river water in northern China. Ecol. Eng. 2011, 37, 560–568. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, J. Improving Winter Performance of Constructed Wetlands for Wastewater Treatment in Northern China: A Review. Wetlands 2014, 34, 243–253. [Google Scholar] [CrossRef]
- Tang, M.; Li, Z.; Yang, Y.; Chen, J.; Jiang, J. Effects of the inclusion of a mixed Psychrotrophic bacteria strain for sewage treatment in constructed wetland in winter seasons. R. Soc. Open Sci. 2018, 5, 172360. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yang, J.; Bai, S.; Ma, F.; Wang, L. Microbial population dynamics in response to bioaugmentation in a constructed wetland system under 10 °C. Bioresour. Technol. 2016, 205, 166–173. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Ma, Y.; Wu, X.; Yang, H. Characterization of nitrification and microbial community in a shallow moss con structed wetland at cold temperatures. Ecol. Eng. 2012, 42, 124–129. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Y.; Wang, Y.; Song, X.; Ambrose, R.F.; Ullman, J.L. Intensified nitrogen removal in immobilized nitrifier en hanced constructed wetlands with external carbon addition. Bioresour. Technol. 2016, 218, 1261–1265. [Google Scholar] [CrossRef]
- Dierberg, F.E.; DeBusk, T.A.; Jackson, S.D.; Chimney, M.J.; Pietro, K. Submerged aquatic vegetation-based treatment wetlands for removing phosphorus from agricultural runoff: Response to hydraulic and nutrient loading. Water Res. 2002, 36, 1409–1422. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, Z.; Tian, B. Nitrate removal by microbial enhancement in a riparian wetland. Bioresour. Technol. 2010, 101, 5712–5718. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Pei, H.Y.; Hu, W.R. Nitrogen removal by bioaugmentation in constructed wetlands for rural domestic wastewater in autumn. Desalination Water Treat. 2013, 51, 6624–6634. [Google Scholar] [CrossRef]
- Liu, F.; Fiencke, C.; Guo, J.; Lyu, T.; Dong, R.; Pfeiffer, E. Optimisation of bioscrubber systems to simultaneously remove me thane and purify wastewater from intensive pig farms. Environ. Sci. Pollut. Res. 2019, 26, 15847–15856. [Google Scholar] [CrossRef]
- Ji, M.; Hu, Z.; Hou, C.; Liu, H.; Ngo, H.H.; Guo, W.; Lu, S.; Zhang, J. New insights for enhancing the performance of constructed wetlands at low temperatures. Bioresour. Technol. 2020, 301, 122722. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Z.; Sun, M. Pollutant removal from municipal sewage in winter via a modified free-water-surface system planted with edible vegetable. Desalination. 2010, 250, 158–161. [Google Scholar] [CrossRef]
- Wang, P.H.; Jeelani, N.; Zuo, J.; Zhang, H.; Zhao, D.H.; Zhu, Z.J.; Leng, X.; An, S.Q. Nitrogen removal during the cold season by constructed floating wetlands planted with Oenanthe javanica. Mar. Freshwater Res. 2018, 69, 635–647. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Wang, W.; Hu, Z.; Yin, X.; Ngo, H.H.; Guo, W.; Fan, J. Enhancement of surface flow constructed wetlands performance at low temperature through seasonal plant collocation. Bioresour. Technol. 2017, 224, 222–228. [Google Scholar] [CrossRef]
- Liu, L.; Li, N.; Tao, C.; Zhao, Y.; Gao, J. Nitrogen removal performance and bacterial communities in zeolite trickling filter under different influent C/N ratios. Environ. Sci. Pollut. Res. 2021, 28, 15909–15922. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.; Guo, X.; Zhu, S.; Chen, S.; Zhang, R. Nutrient removal capability and growth characteristics of Iris sibirica in subsurface vertical flow constructed wetlands in winter. Ecol. Eng. 2014, 70, 351–361. [Google Scholar] [CrossRef]
- Matysek, M.; Leake, J.; Banwart, S.; Johnson, I.; Page, S.; Kaduk, J.; Smalley, A.; Cumming, A.; Zona, D. Impact of fertiliser, water table, and warming on celery yield and CO2 and CH4 emissions from fenland agricultural peat. Sci. Total. Environ. 2019, 667, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Das, A.; Jha, P.; Jha, P.N. Endophytic Bacteria Pseudomonas aeruginosa PM389 Subsists Host’s (Triticum aestivum) Immune Response for Gaining Entry Inside the Host. J. Pure Appl. Microbiol. 2021, 15, 2486–2497. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Islam, M.M.; Wang, R.; Guo, J.; Luo, H.; Chen, F.; Li, X. Glutamine application promotes nitrogen and biomass accumulation in the shoot of seedlings of the maize hybrid ZD958. Planta 2020, 251, 1–15. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Chang, A.C.; Arshad, M. Response of Raphanus sativus to the auxin precursor, L-tryptophan applied to soil. Plant. Soil 1990, 129, 235–241. [Google Scholar] [CrossRef]
- Huang, J.; Peng, Y.; Chung, K.; Huang, J. Suppressive efficacy of volatile compounds produced by Bacillus mycoides on damp ing-off pathogens of cabbage seedlings. J. Agric. Sci. 2018, 156, 795–809. [Google Scholar] [CrossRef]
- Petersen, D.J.; Shishido, M.; Holl, F.B.; Chanway, C.P. Use of species- and strain-specific PCR primers for identification of conifer root-associated Bacillus spp. FEMS Microbiol. Lett. 1995, 133, 71–76. [Google Scholar] [CrossRef]
- Lu, H.; Xiao, L.; Wang, T.; Lu, S.; Wang, H.; Guo, X.; Li, J. The application of steel slag in a multistage pond constructed wetland to purify low-phosphorus polluted river water. J. Environ. Manag. 2021, 292, 112578. [Google Scholar] [CrossRef]
- Barca, C.; Meyer, D.; Liira, M.; Drissen, P.; Comeau, Y.; Andrès, Y.; Chazarenc, F. Steel slag filters to upgrade phosphorus re moval in small wastewater treatment plants: Removal mechanisms and performance. Ecol. Eng. 2014, 68, 214–222. [Google Scholar] [CrossRef]
- Zhu, T.; Gao, J.; Huang, Z.; Shang, N.; Gao, J.; Zhang, J.; Cai, M. Comparison of performance of two large-scale vertical-flow constructed wetlands treating wastewater treatment plant tail-water: Contaminants removal and associated microbial community. J. Environ. Manag. 2021, 278, 111564. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.Q.; Liu, R.B.; Morgan, D.; Wei, T. Enhancing wastewater remediation by drinking water treatment residual-augmented floating treatment wetlands. Sci. Total. Environ. 2019, 673, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, A.O.; Zhao, Y.Q.; Doyle, R.J.; Rackard, S.M.; Kumar, J.L.G.; Hu, Y.S. Performance evaluation and prediction for a pilot two-stage on-site constructed wetland system employing dewatered alum sludge as main substrate. Bioresour. Technol. 2011, 102, 5645–5652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Zhang, J.; Wang, S.; Liang, S.; Hu, Z. Enhanced phosphorus removal of constructed wetland through plant growth-promoting rhizobacteria (PGPR) addition. Environ. Sci. Pollut. Res. 2021, 28, 52124–52132. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total. Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Scholz, M.; Bouillon, L. Seasonal assessment of experimental vertical-flow constructed wetlands treating domestic wastewater. Bioresour. Technol. 2013, 147, 585–596. [Google Scholar] [CrossRef]
- Guan, B.; Yao, X.; Jiang, J.; Tian, Z.; An, S.; Gu, B.; Cai, Y. Phosphorus removal ability of three inexpensive substrates: Physico chemical properties and application. Ecol. Eng. 2009, 35, 576–581. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Gao, J.; Huang, Z.; Zhou, H.; Duan, H.; Zhang, Z. Preparation of a novel non-burning polyaluminum chloride residue (PACR) compound filler and its phosphate removal mechanisms. Environ. Sci. Pollut. Res. 2021, 29, 1532–1545. [Google Scholar] [CrossRef]
- Jena, J.; Kumar, R.; Saifuddin, M.; Dixit, A.; Das, T. Anoxic–aerobic SBR system for nitrate, phosphate and COD removal from high-strength wastewater and diversity study of microbial communities. Biochem. Eng. J. 2016, 105, 80–89. [Google Scholar] [CrossRef]
- Fouts, D.E.; Szpakowski, S.; Purushe, J.; Torralba, M.; Waterman, R.C.; MacNeil, M.D.; Alexander, L.J.; Nelson, K.E. Next gen eration sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS ONE 2012, 7, e48289. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Li, M.; Wang, H.; Li, Y.; Peng, H.; Feng, J. Microbial community and function evaluation in the start-up period of bioaugmented SBR fed with aniline wastewater. Bioresour. Technol. 2021, 319, 124148. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, H.; Chen, X.; Wang, R.; Liu, J. Composition and distribution of microbial communities in natural river wetlands and corresponding constructed wetlands. Ecol. Eng. 2017, 98, 40–48. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Ham, J.H. Comparative analysis of the microbial community in the sediments of two constructed wetlands differ entially influenced by the concentrated poultry feeding operations. J. Soils Sediments 2017, 17, 557–566. [Google Scholar] [CrossRef]
- Yan, Q.; Min, J.; Yu, Y.; Zhu, Z.; Feng, G. Microbial community response during the treatment of pharmaceutically active com pounds (PhACs) in constructed wetland mesocosms. Chemosphere 2017, 186, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, C.; Zhang, X.; Song, X. Addition of iron materials for improving the removal efficiencies of multiple contaminants from wastewater with a low C/N ratio in constructed wetlands at low temperatures. Environ. Sci. Pollut. Res. 2019, 26, 11988–11997. [Google Scholar] [CrossRef] [PubMed]
- Yavuztürk Gül, B.; Imer, D.Y.; Park, P.; Koyuncu, I. Evaluation of a novel anti-biofouling microorganism (Bacillus sp. T5) for control of membrane biofouling and its effect on bacterial community structure in membrane bioreactors. Water Sci. Technol. 2018, 77, 971–978. [Google Scholar] [CrossRef]
- Ravcheev, D.A.; Gerasimova, A.V.; Mironov, A.A.; Gelfand, M.S. Comparative genomic analysis of regulation of anaerobic res piration in ten genomes from three families of gamma-proteobacteria (Enterobacteriaceae, Pasteurellaceae, Vibrionaceae). BMC Genom. 2007, 8, 54. [Google Scholar] [CrossRef]
- Anzai, Y.; Kim, H.; Park, J.Y.; Wakabayashi, H.; Oyaizu, H. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 2000, 50, 1563–1589. [Google Scholar] [CrossRef] [Green Version]










| Sample ID | Sequences | OTUs | ACE | Chao | Shannon |
|---|---|---|---|---|---|
| A1 | 111,713 | 3648 | 3672.31 | 3651.90 | 9.50 |
| A2 | 105,146 | 3753 | 4988.66 | 4988.66 | 9.79 |
| B1 | 105,501 | 3604 | 3569.52 | 3569.52 | 9.50 |
| B2 | 116,209 | 3389 | 3354.47 | 3354.47 | 9.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Li, Q.; Zhang, J.; Wang, S.; Song, B.; Huang, Z. Purification of Micro-Polluted Lake Water by Biofortification of Vertical Subsurface Flow Constructed Wetlands in Low-Temperature Season. Water 2022, 14, 896. https://doi.org/10.3390/w14060896
Gao J, Li Q, Zhang J, Wang S, Song B, Huang Z. Purification of Micro-Polluted Lake Water by Biofortification of Vertical Subsurface Flow Constructed Wetlands in Low-Temperature Season. Water. 2022; 14(6):896. https://doi.org/10.3390/w14060896
Chicago/Turabian StyleGao, Jingqing, Qiang Li, Jingshen Zhang, Shilong Wang, Bozhen Song, and Zhenzhen Huang. 2022. "Purification of Micro-Polluted Lake Water by Biofortification of Vertical Subsurface Flow Constructed Wetlands in Low-Temperature Season" Water 14, no. 6: 896. https://doi.org/10.3390/w14060896