Removal of Phosphorus from Hypolimnetic Lake Water by Reactive Filter Material in a Recirculating System—Laboratory Trial
Abstract
:1. Introduction
2. Materials and Methods
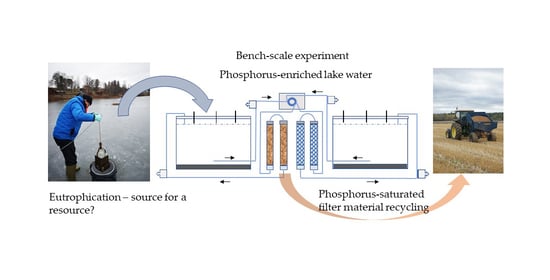
2.1. Experimental Set-Up
2.2. Collection of Water and Sediment from Lake Hönsan
2.3. Analyses and P Removal Calculation
3. Results and Discussion
3.1. Phosfate Removal in the Columns and Changes in PO4-P Concentration in the Containers
3.2. Changes in Physio-Chemical Conditions of the Water during Pumping
3.3. Problems Needing Further Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noges, P.; Van De Bund, W.; Cardoso, A.C.; Solimini, A.G.; Heiskanen, A.S. Assessment of the ecological status of European surface waters: A work in progress. Hydrobiologia 2009, 633, 197–211. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Rydin, E.; Welch, E.B. Aluminum dose required to inactivate phosphate in lake sediments. Water Res. 1998, 32, 2969–2976. [Google Scholar] [CrossRef]
- Rydin, E.; Kumblad, L. Capturing past eutrophication in coastal sediments—Towards water-quality goals. Estuar. Coast. Shelf Sci. 2019, 221, 184–188. [Google Scholar] [CrossRef]
- Dithmer, L.; Nielsen, U.G.; Lürling, M.; Spears, B.M.; Yasseri, S.; Lundberg, D.; Moore, A.; Jensen, N.D.; Reitzel, K. Responses in sediment phosphorus and lanthanum concentrations and composition across 10 lakes following applications of lanthanum modified bentonite. Water Res. 2016, 7, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dondajewska, R.; Kowalczewska-Madura, K.; Gołdyn, R.; Kozak, A.; Messyasz, B.; Cerbin, S. Long-term water quality changes as a result of a sustainable restoration—A case study of dimictic Lake Durowskie. Water 2019, 11, 616. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.I.; Blowes, D.W.; Ptacek, C.J.; Olding, D. Phosphorus removal from lake water using basic oxygen furnace slag: System performance and characterization of reaction products. Environ. Eng. Sci. 2014, 31, 631–642. [Google Scholar] [CrossRef]
- Łożyńska, J.; Dunalska, J.A.; Bańkowska-Sobczak, A.; Zhang, L.; Mitsch, W.J. Treatment of Hypolimnion Water on Mineral Aggregates as the Second Step of the Hypolimnetic Withdrawal Method Used for Lake Restoration. Minerals 2021, 11, 98. [Google Scholar] [CrossRef]
- Łożyńska, J.; Bańkowska-Sobczak, A.; Popek, Z.; Dunalska, J.A. Selection of P-reactive materials for treatment of hypolimnetic water withdrawn from eutrophic lakes. Ecohydr. Hydrobiol. 2020, 20, 276–288. [Google Scholar] [CrossRef]
- Silvonen, S.; Niemistö, J.; Csibrán, A.; Jilbert, T.; Torma, P.; Krámer, T.; Nurminen, L.; Horppila, J. A biogeochemical approach to evaluate the optimization and effectiveness of hypolimnetic withdrawal. Sci. Total Environ. 2021, 755, 143202. [Google Scholar] [CrossRef]
- Nürnberg, G.K.; Hartley, R.; Davis, E. Hypolimnetic withdrawal in two North American lakes with anoxic phosphorus release from the sediment. Water Res. 1987, 21, 923–928. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Lake responses to long-term hypolimnetic withdrawal treatments. Lake Reserv. Manag. 2007, 23, 388–409. [Google Scholar] [CrossRef] [Green Version]
- Zamparas, M.; Zacharias, I. Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci. Total Environ. 2014, 496, 551–562. [Google Scholar] [CrossRef]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 on establishing a framework for community action in the field of water policy. J. Eur. Commun. 2000, L327, 1–72. [Google Scholar]
- Cucarella, V.; Mazurek, R.; Zaleski, T.; Kopeć, M.; Renman, G. Effect of Polonite used for phosphorus removal from wastewater on soil properties and fertility of a mountain meadow. Environ. Pollut. 2009, 157, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Montaño, V.; Fenton, O.; Moore, B.; Healy, M.G. Evaluation of the fertiliser replacement value of phosphorus-saturated filter media. J. Clean. Prod. 2021, 291, 125943. [Google Scholar] [CrossRef]
- Patyal, V.; Jaspal, D.; Khare, K. Materials for phosphorous remediation: A review. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 1025–1037. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Woja, K.; Bliska, P.; Baryła, A.; Bus, A. The efficiency of filtration materials Polonite® and Leca® supporting phosphorus removal in on site treatment systems with wastewater infiltration. Infrastruktura i Ekologia Terenów Wiejskich 2017, 4, 1401–1413. [Google Scholar] [CrossRef]
- Fastner, J.; Abella, S.; Litt, A.; Morabito, G.; Matthews, D.; Phillips, M.G.; Chorus, I. Combating cyanobacterial proliferation by avoiding or treating inflows with high P load—Experiences from eight case studies. Aquat. Ecol. 2016, 50, 367–383. [Google Scholar] [CrossRef] [Green Version]
- Renman, A.; Renman, G. Long-term phosphate removal by the calcium-silicate material Polonite in wastewater filtration systems. Chemosphere 2010, 79, 659–664. [Google Scholar] [CrossRef]
- Vidal, B.; Hedström, A.; Herrmann, I. Phosphorus reduction in filters for on-site wastewater treatment. J. Water Process Eng. 2018, 22, 210–217. [Google Scholar] [CrossRef]
- Hamisi, R.; Renman, G.; Renman, A.; Wörman, A. Modelling phosphorus sorption kinetics and the longevity of reactive filter materials used for on-site wastewater treatment. Water 2019, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- Brogowski, Z.; Renman, G. Characterization of opoka as a basis for its use in wastewater treatment. Pol. J. Environ. Stud. 2004, 13, 15–20. [Google Scholar]
- Renman, A. On-Site Wastewater Treatment: Polonite and Other Filter Materials for Removal of Metals, Nitrogen and Phosphorus. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2008. Trita-LWR.PHD, 1043. Available online: http://kth.diva-portal.org/smash/record.jsf?pid=diva2:14097 (accessed on 4 February 2022).
- Eveborn, D.; Gustafsson, J.P.; Hesterberg, D.; Hillier, S. XANES speciation of P in environmental samples: An assessment of filter media for on-site wastewater treatment. Environ. Sci. Technol. 2009, 43, 6515–6521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolosov, P.V. Chemistry of Phosphorus Removal by Polonite® Media. Ph.D. Thesis, Tennessee Technological University, Cookeville, TN, USA, 2014. [Google Scholar]
- Rubulis, J.; Juhna, T. Evaluating the potential of biofilm control in water supply systems by removal of phosphorus from drinking water. Water Sci. Technol. 2007, 55, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Xing, X.H.; Wang, B.Z. Characteristics of phosphorus removal from wastewater by biofilm sequencing batch reactor (SBR). Biochem. Eng. J. 2003, 16, 279–285. [Google Scholar] [CrossRef]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef]
- Cucarella, V.; Renman, G. Phosphorus sorption capacity of filter materials used for on-site wastewater treatment determined in batch experiments—A comparative study. J. Environ. Qual. 2009, 38, 381–392. [Google Scholar] [CrossRef]
- Nilsson, C.; Lakshmanan, R.; Renman, G.; Rajarao, G.K. Efficacy of reactive mineral-based sorbents for phosphate, bacteria, nitrogen and TOC removal–column experiment in recirculation batch mode. Water Res. 2013, 47, 5165–5175. [Google Scholar] [CrossRef] [Green Version]
- Courcelles, B.; Modaressi-Farahmand-Razavi, A.; Gouvenot, D.; Esnault-Filet, A. Influence of precipitates on hydraulic performance of permeable reactive barrier filters. Int. J. Geomech. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Wikström, J.; Bonaglia, S.; Ramö, R.; Renman, G.; Walve, J.; Hedberg, J.; Gunnarsson, J.S. Sediment remediation with new composite sorbent amendments to sequester phosphorus, organic contaminants, and metals. Environ. Sci. Technol. 2021, 55, 11937–11947. [Google Scholar] [CrossRef] [PubMed]
- Tammeorg, O.; Nürnberg, G.; Nõges, P.; Niemistö, J. Sediment phosphorus release in boreal lakes: The role of trophic state and humic substances. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Alvarez, R.; Evans, L.A.; Milham, P.J.; Wilson, M.A. Effects of humic material on the precipitation of calcium phosphate. Geoderma 2004, 118, 245–260. [Google Scholar] [CrossRef]
- Reddy, M.M.; Hoch, A.R. Calcite crystal growth rate inhibition by aquatic humic substances. In Advances in Crystal Growth Inhibition Technologies; Springer: Boston, MA, USA, 2002; pp. 107–121. [Google Scholar]
- Boström, B. Relations between chemistry, microbial biomass and activity in sediments of a polluted vs. a non-polluted eutrophic lake. Int. Ver. Für Theor. Angew. Limnol. Verh. 1988, 23, 451–459. [Google Scholar]
- Qu, J.H.; Li, H.F.; Chen, N.; Yuan, H.L. Biogeochemical function of phosphorus-solubilising bacteria on cycling of phosphorus at the water-sediment interface under laboratorial simulated conditions. Int. J. Environ. Pollut. 2013, 52, 104–116. [Google Scholar] [CrossRef]
- Qian, Y.; Liang, X.; Chen, Y.; Lou, L.; Cui, X.; Tang, J.; Li, P.; Cao, R. Significance of biological effects on phosphorus transformation processes at the water–sediment interface under different environmental conditions. Ecol. Eng. 2011, 37, 816–825. [Google Scholar] [CrossRef]
- Christophoridis, C.; Fytianos, K. Conditions affecting the release of phosphorus from surface lake sediments. J. Environ. Qual. 2006, 35, 1181–1192. [Google Scholar] [CrossRef]
- Ortuno, J.F.; Saez, J.; Llorens, M.; Soler, A. Phosphorus release from sediments of a deep wastewater stabilization pond. Water Sci. Technol. 2000, 42, 265–272. [Google Scholar] [CrossRef]
- Mayes, W.M.; Batty, L.C.; Younger, P.L.; Jarvis, A.P.; Kõiv, M.; Vohla, C.; Mander, U. Wetland treatment at extremes of pH: A review. Sci. Total Environ. 2009, 407, 3944–3957. [Google Scholar] [CrossRef]
- Renman, G.; Renman, A. Sustainable use of crushed autoclaved aerated concrete (CAAC) as a filter medium in wastewater purification. In Proceedings of the 8th International Conference on Sustainable Management of Waste and Recycled Materials in Construction, Gothenburg, Sweden, 30 May–1 June 2012. ISCOWA and SGI. [Google Scholar]
- Tandyrak, R.; Gołaś, I.; Parszuto, K.; Bowszys, M.; Szymański, D.; Harnisz, M.; Brudniak, A.; Wysocka, I. The effect of lake restoration by the hypolimnetic withdrawal method on the intensity of ambient odour. J. Limnol. 2016, 75, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Fronczyk, J.; Mumford, K.A. The impact of temperature on the removal of inorganic contaminants typical of urban stormwater. Appl. Sci. 2019, 9, 1273. [Google Scholar] [CrossRef] [Green Version]
- Benzing, S.; Couceiro, F.; Barnett, S.; Williams, J.B.; Pearce, P.; Stanford, C. Impact of hydraulic retention time on phosphorus removal from wastewater using reactive media. Water Sci. Technol. 2020, 82, 2920–2928. [Google Scholar] [CrossRef]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A review of phosphorus removal structures: How to assess and compare their performance. Water 2017, 9, 583. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for recovering nutrients from wastewater: A critical review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R. Cost–benefit analysis of water-reuse projects for environmental purposes: A case study for Spanish wastewater treatment plants. J. Environ. Manag. 2011, 92, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Braga, B.B.; de Carvalho, T.R.A.; Brosinsky, A.; Foerster, S.; Medeiros, P.H.A. From waste to resource: Cost-benefit analysis of reservoir sediment reuse for soil fertilization in a semiarid catchment. Sci. Total Environ. 2019, 670, 158–169. [Google Scholar] [CrossRef]
- Bashar, R.; Gungor, K.; Karthikeyan, K.G.; Barak, P. Cost effectiveness of phosphorus removal processes in municipal wastewater treatment. Chemosphere 2018, 197, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Barquet, K.; Järnberg, L.; Rosemarin, A.; Macura, B. Identifying barriers and opportunities for a circular phosphorus economy in the Baltic Sea region. Water Res. 2020, 171, 115433. [Google Scholar] [CrossRef] [PubMed]


| Chemical */Physical Parameter | Value |
|---|---|
| Quartz, SiO2 (%) | 50.5 ± 4.1 ** |
| Calcium oxide, CaO (%) | 40.1 ± 3.6 |
| Aluminium oxide, Al2O3 (%) | 4.2 ± 1.8 |
| Ferric oxide, Fe2O3 (%) | 2.6 ± 1.3 |
| Wollastonite (CaSiO3) (%) | 2.1 ± 0.7 |
| pH *** | 12.3 ± 0.1 |
| Particle size (mm) | 1–4 |
| Density (g cm−3) | 0.81 ± 0.03 |
| Bed Volume | Polonite C. Influent (µg L−1) | Polonite C. Effluent (µg L−1) | Glass bead C. Influent (µg L−1) | Glass bead C. Effluent (µg L−1) | Removal (%) | |
|---|---|---|---|---|---|---|
| Polonite | Glass Bead | |||||
| 7.5 | 380.16 | 0 | 390.10 | 380.50 | 100 | 2.5 |
| 22.5 | 383.25 | 0 | 399.35 | 405.31 | 100 | −1.5 * |
| 45 | 381.30 | 0 | 403.13 | 411.86 | 100 | −2.2 * |
| 70 | 373.19 | 0 | 392.52 | 360.35 | 100 | 8.2 |
| 90.5 | 388.55 | 0 | 402.35 | 389.11 | 100 | 3.3 |
| 113 | 387.32 | 0 | 406.11 | 392.31 | 100 | 3.4 |
| 135.5 | 389.01 | 0.40 | 401.52 | 386.52 | 99.9 | 3.7 |
| 165.5 | 390.25 | 0.74 | 409.33 | 391.52 | 99.8 | 4.4 |
| 188 | 375.52 | 4.39 | 412.10 | 400.22 | 98.8 | 2.9 |
| 210.5 | 320.25 | 7.36 | 399.32 | 391.15 | 97.7 | 2.1 |
| 233 | 280.19 | 14.13 | 401.67 | 392.35 | 95 | 2.3 |
| 240 | 255.16 | 20.52 | 395.29 | 387.57 | 92 | 2 |
| Average | 358.68 | 3.96 | 401.07 | 390.73 | 98.60 | 2.9 |
| St. dev. | 48.53 | 6.99 | 5.69 | 13.03 | 2.65 | 2.79 |
| Bed Volume Treated and pH, Container A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bed volume | 7.5 | 22.5 | 45 | 70 | 90.5 | 113 | 135.5 | 165.5 | 188 | 210.5 | 233 | 240 |
| Inflow | 12.1 | 11.9 | 11.8 | 11.7 | 11.6 | 11.5 | 11.4 | 11.4 | 11.1 | 10.9 | 10.9 | 10.8 |
| Surface | 8.1 | 8.7 | 9.1 | 9.3 | 8.4 | 8.8 | 8.3 | 7.8 | 7.9 | 7.6 | 8.5 | 8.6 |
| Middle | 7.7 | 7.9 | 9.4 | 9.5 | 8.6 | 8.7 | 8.8 | 8.2 | 8.0 | 8.1 | 8.6 | 8.5 |
| Bottom | 7.1 | 9.1 | 9.6 | 9.7 | 9.3 | 9.5 | 9.4 | 8.8 | 9.1 | 9.1 | 9.3 | 8.8 |
| Bed volume treated and pH, Container B | ||||||||||||
| Bed volume | 7.5 | 22.5 | 45 | 70 | 90.5 | 113 | 135.5 | 165.5 | 188 | 210.5 | 233 | 240 |
| Inflow | 6.7 | 6.9 | 7.0 | 7.2 | 7.2 | 7.1 | 7.3 | 6.9 | 6.8 | 7.1 | 6.7 | 6.8 |
| Surface | 7.1 | 6.8 | 6.9 | 7.3 | 7.0 | 6.9 | 7.2 | 6.8 | 6.7 | 6.9 | 6.7 | 6.7 |
| Middle | 6.9 | 7.3 | 7.2 | 7.1 | 7.3 | 7.1 | 6.9 | 7.0 | 6.9 | 6.8 | 6.8 | 6.6 |
| Bottom | 6.6 | 6.7 | 6.5 | 6.5 | 6.6 | 6.4 | 6.6 | 6.5 | 6.6 | 6.8 | 6.7 | 6.7 |
| Bed Volume Treated and DO (mg L−1), Container A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bed volume | 7.5 | 22.5 | 45 | 70 | 90.5 | 113 | 135.5 | 165.5 | 188 | 210.5 | 233 | 240 |
| Inflow | 4.33 | 2.25 | 2.14 | 1.35 | 1.24 | 1.05 | 1.11 | 0.97 | 1.01 | 0.95 | 0.98 | 0.96 |
| Surface | 1.33 | 1.24 | 1.30 | 1.10 | 1.11 | 1.02 | 0.99 | 0.93 | 0.95 | 0.93 | 0.97 | 0.99 |
| Middle | 0.76 | 0.82 | 0.84 | 0.71 | 0.63 | 0.65 | 0.67 | 0.59 | 0.64 | 0.65 | 0.71 | 0.76 |
| Bottom | 0.11 | 0.15 | 0.14 | 0.15 | 0.16 | 0.10 | 0.12 | 0.14 | 0.14 | 0.18 | 0.23 | 0.25 |
| Average | 0.73 | 0.74 | 0.76 | 0.65 | 0.63 | 0.59 | 0.59 | 0.55 | 0.58 | 0.59 | 0.64 | 0.66 |
| Bed volume treated and DO (mg L−1), Container B | ||||||||||||
| Bed volume | 7.5 | 22.5 | 45 | 70 | 90.5 | 113 | 135.5 | 165.5 | 188 | 210.5 | 233 | 240 |
| Inflow | 5.23 | 3.22 | 2.35 | 2.21 | 1.85 | 1.56 | 1.48 | 1.47 | 1.45 | 1.48 | 1.50 | 1.45 |
| Surface | 3.51 | 2.95 | 2.11 | 1.90 | 1.55 | 1.26 | 1.19 | 1.20 | 1.13 | 1.05 | 0.98 | 0.97 |
| Middle | 2.10 | 1.93 | 1.35 | 1.27 | 1.11 | 0.90 | 0.92 | 0.90 | 0.87 | 0.81 | 0.83 | 0.86 |
| Bottom | 0.15 | 0.14 | 0.14 | 0.17 | 0.15 | 0.17 | 0.13 | 0.12 | 0.15 | 0.15 | 0.17 | 0.15 |
| Average | 1.92 | 1.67 | 1.20 | 1.11 | 0.94 | 0.78 | 0.75 | 0.74 | 0.72 | 0.67 | 0.66 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renman, A.; Renman, G. Removal of Phosphorus from Hypolimnetic Lake Water by Reactive Filter Material in a Recirculating System—Laboratory Trial. Water 2022, 14, 819. https://doi.org/10.3390/w14050819
Renman A, Renman G. Removal of Phosphorus from Hypolimnetic Lake Water by Reactive Filter Material in a Recirculating System—Laboratory Trial. Water. 2022; 14(5):819. https://doi.org/10.3390/w14050819
Chicago/Turabian StyleRenman, Agnieszka, and Gunno Renman. 2022. "Removal of Phosphorus from Hypolimnetic Lake Water by Reactive Filter Material in a Recirculating System—Laboratory Trial" Water 14, no. 5: 819. https://doi.org/10.3390/w14050819