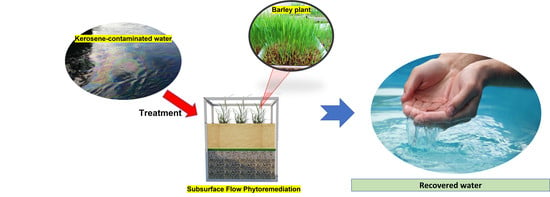
Subsurface Flow Phytoremediation Using Barley Plants for Water Recovery from Kerosene-Contaminated Water: Effect of Kerosene Concentration and Removal Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary Test
2.2. Setup of the Phytoremediation Experimental Test Procedure
2.3. The Measurement of Water Physicochemical Properties
2.4. Water Sampling
2.5. Sand Sampling
2.6. Plant Growth
2.7. Analysis and Removal Percentage of Kerosene
3. Results and Discussion
3.1. Preliminary Test
3.2. Monitoring of Physicochemical Parameters
3.3. Degradation and Removal of Kerosene from Water
3.4. Removal of Kerosene in Sand
3.5. Plant Behaviour to the Kerosene Contaminant
3.6. Kinetics of Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moubasher, H.; Hegazy, A.; Mohamed, N.; Moustafa, Y.; Kabiel, H.; Hamad, A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Ghoreishi, G.; Alemzadeh, A.; Mojarrad, M.; Djavaheri, M. Bioremediation capability and characterization of bacteria isolated from petroleum contaminated soils in Iran. Sustain. Environ. Res. 2017, 27, 195–202. [Google Scholar] [CrossRef]
- Sharonova, N.; Breus, I. Tolerance of cultivated and wild plants of different taxonomy to soil contamination by kerosene. Sci. Total Environ. 2012, 424, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Mahyar, A.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Comparative study on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J. Hazard. Mater. 2018, 343, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Haan, T.Y.; Fen, C.S.; Radzi, M.F.; Ganasen, U. Comparative study for lake water remediation: Chemical coagulation and electrocoagulation. J. Kej. SI 2018, 1, 81–87. [Google Scholar]
- Tursi, A.; De Vietro, N.; Beneduci, A.; Milella, A.; Chidichimo, F.; Fracassi, F. Low pressure plasma functionalized cellulose fiber for the remediation of petroleum hydrocarbons polluted water. J. Hazard. Mater. 2019, 373, 773–782. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Al-Haleem, A.A.; Abed, K.M. Treating of oil-based drill cuttings by earthworms. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2088–2094. [Google Scholar]
- Zahed, M.A.; Salehi, S.; Madadi, R.; Hejabi, F. Biochar as a sustainable product for remediation of petroleum contaminated soil. Curr. Res. Green Sustain. Chem. 2021, 4, 100055. [Google Scholar] [CrossRef]
- Yusoff, M.F.M.; Abdullah, S.R.S.; Hasan, H.A. Performance of continuous pilot subsurface constructed wetland using Scirpus grossus for removal of COD, colour and suspended solid in recycled pulp and paper effluent. Environ. Technol. Innov. 2019, 13, 346–352. [Google Scholar] [CrossRef]
- Ismail, N.I.; Abdullah, S.R.S.; Idris, M.; Kurniawan, S.B.; Effendi, M.I.; ALSbani, N.H.; Jehawi, O.H.; Hasan, H.A. Applying rhizobacteria consortium for the enhancement of Scirpus grossus growth and phytoaccumulation of Fe and Al in pilot constructed wetlands. J. Environ. Manag. 2020, 267, 110643. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.C.; Vessoni-Penna, T.C.; de Souza Oliveira, R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Zeki, S.L.; M-Ridha, M.J. Phytoremediation of synthetic wastewater containing copper by using native plant. Iraqi J. Agric. Sci. 2020, 51, 1601–1612. [Google Scholar]
- Al-Baldawi, I.; Abdullah, S.R.S.; Anuar, N.; Suja, F.; Mushrifah, I. Phytodegradation of total petroleum hydrocarbon (TPH) in diesel-contaminated water using Scirpus grossus. Ecol. Eng. 2015, 74, 463–473. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: Application, mechanisms, challenges and opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Idris, M.; Abdullah, S.R.S.; Anuar, N.; Kurniawan, S.B. Response and capability of Scirpus mucronatus (L.) in phytotreating petrol-contaminated soil. Chemosphere 2021, 269, 128760. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, Q.; Yu, B. Responses and roles of roots, microbes, and degrading genes in rhizosphere during phytoremediation of petroleum hydrocarbons contaminated soil. Int. J. Phytoremed. 2019, 21, 1161–1169. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Powell, J.; Singh, H.B.; Singh, B. Plant–microbe interactions: Novel applications for exploitation in multipurpose remediation technologies. Trends Biotechnol. 2012, 30, 416–420. [Google Scholar] [CrossRef]
- Ali, M.F.; M-Ridha, M.J.; Taly, A.H. Optimization kerosene bio-degradation by a local soil bacterium isolate klebsiella pneumonia sp. pneumonia. J. Pure Appl. Microbiol. 2018, 12, 2019–2057. [Google Scholar]
- Wojtera-Kwiczor, J.; Zukowska, W.; Graj, W.; Małecka, A.; Piechalak, A.; Ciszewska, L.; Chrzanowski, Ł.; Lisiecki, P.; Ko-morowicz, I.; Barałkiewicz, D.; et al. Rhizoremediation of diesel-contaminated soil with two rapeseed variaties and petroleum degraders reveals different responses of the plant defense mechanisms. Int. J. Phytorem. 2014, 16, 770–789. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, F.J.; Cañizares, P.; Rodríguez, L. Assessing the phytoremediation potential of crop and grass plants for atrazine-spiked soils. Chemosphere 2017, 185, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathod, P.H.; Brackhage, C.; Van Der Meer, F.D.; Müller, I.; Noomen, M.; Rossiter, D.G.; Dudel, G.E. Spectral changes in the leaves of barley plant due to phytoremediation of metals—Results from a pot study. Eur. J. Remote Sens. 2015, 48, 283–302. [Google Scholar] [CrossRef]
- González, A.; Gil-Díaz, M.M.; Pinilla, P.; Lobo, M.C. Impact of Cr and Zn on Growth, Biochemical and Physiological Parameters, and Metal Accumulation by Wheat and Barley Plants. Water Air Soil Pollut. 2017, 228, 419. [Google Scholar] [CrossRef]
- Al-Magheer, H.A.M.; Aldahak, L.; Al-Sali, S.H.F. Evaluation of growth and productivity of barley (Hordeum vulgare L.) genotype under southern Iraq conditions. Int. J. Plant Res. 2020, 21, 132–136. [Google Scholar]
- Kreszies, T.; Schreiber, L.; Ranathunge, K. Suberized transport barriers in Arabidopsis, barley and rice roots: From the model plant to crop species. J. Plant Physiol. 2018, 227, 75–83. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Introduction to Phytoremediation. EPA 600-R-99-107; 2000. Available online: http://cluin.org/download/remed/introphyto.pdf (accessed on 3 September 2010).
- United States Environmental Protection Agency. Method 3510C. Separatory Funnel Liquid-Liquid Extraction. 2011. Available online: http://www.caslab.com/EPA-Methods/PDF/EPA-Method-3510C.pdf (accessed on 5 June 2012).
- Lohi, A.; Cuenca, M.A.; Anania, G.; Upreti, S.; Wan, L. Biodegradation of diesel fuel-contaminated wastewater using a three-phase fluidized bed reactor. J. Hazard. Mater. 2008, 154, 105–111. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Method 3550C, Ultrasonic Extraction, SW-846 Manual; U.S. Government Printing Office: Washington, DC, USA, 2007. Available online: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3550c.pdf (accessed on 11 May 2011).
- Liu, X.; Wang, Z.; Zhang, X.; Wang, J.; Xu, G.; Cao, Z.; Zhong, C.; Su, P. Degradation of diesel-originated pollutants in wetlands by Scirpus triqueter and microorganisms. Ecotoxicol. Environ. Saf. 2011, 74, 1967–1972. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, Q.; Cai, Z.; Zhang, Z. Phytoremediation of petroleum contaminated soils by Mirabilis Jalapa L. in a greenhouse plot experiment. J. Hazard. Mater. 2009, 168, 1490–1496. [Google Scholar] [CrossRef]
- Zeki, S.L.; M-Ridha, M.J. Phytotoxicity of wastewater containing cadmium (Cd) using oleander. Iraqi J. Agric. Sci. 2020, 51, 1231–1238. [Google Scholar]
- Barati, M.; Bakhtiari, F.; Mowla, D.; Safarzadeh, S. Comparison of the effects of poultry manure and its biochar on barley growth in petroleum-contaminated soils. Int. J. Phytoremed. 2018, 20, 98–103. [Google Scholar] [CrossRef]
- Asiabadi, F.I.; Mirbagheri, S.A.; Najafi, P.; Moatar, F. Phytoremediation of Petroleum-Contaminated Soils around Isfahan Oil Refinery (Iran) by Sorghum and Barley. Curr. World Environ. 2014, 9, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.-A.; Uchiyama, K.; Inadama, D.; Ishida, Y.; Yamagiwa, K. Treatment of azo dye Acid Orange 7 containing wastewater using up-flow constructed wetland with and without supplementary aeration. Bioresour. Technol. 2010, 101, 9049–9057. [Google Scholar] [CrossRef] [PubMed]
- Shirdam, R.; Zand, A.D.; Bidhendi, G.N.; Mehrdadi, N. Phytoremediation of hydrocarbon-contaminated soils with emphasis on the effect of petroleum hydrocarbons on the growth of plant species. Phytoprotection 2009, 89, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Merkl, N.; Schultze-Kraft, R.; Infante, C. Phytoremediation in the Tropics—The Effect of Crude Oil on the Growth of Tropical Plants. Bioremed. J. 2004, 8, 177–184. [Google Scholar] [CrossRef]
- Liste, H.-H.; Felgentreu, D. Crop growth, culturable bacteria, and degradation of petrol hydrocarbons (PHCs) in a long-term contaminated field soil. Appl. Soil Ecol. 2006, 31, 43–52. [Google Scholar] [CrossRef]
- Bossert, I.; Bartha, R. Plant growth in soils with a history of oily sludge disposal. Soil Sci. 1985, 140, 75–77. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Nwachukwu, E.O.; Sikoki, F.D. Assessing and modelling the efficacy of Lemna paucicostata for the phytoremediation of petroleum hydrocarbons in crude oil-contaminated wetlands. Sci. Rep. 2020, 10, 8489. [Google Scholar] [CrossRef]







| Model | Mathematical Formula |
|---|---|
| Zero order | |
| First order | |
| Second order | |
| Grau |
| System | Model | Parameter | Initial Concentration | ||
|---|---|---|---|---|---|
| 1% | 2% | 3% | |||
| Plant | First order | 0.049 | 0.142 | 0.1535 | |
| R2 | 0.687 | 0.921 | 0.9407 | ||
| RSME | 703 | 1143 | 1713 | ||
| MSE | 494,218 | 1,307,427 | 2,933,396 | ||
| Second order | 0.000033 | 0.000015 | 0.000011 | ||
| R2 | 0.976 | 0.972 | 0.9793 | ||
| RSME | 445 | 721 | 790 | ||
| MSE | 197,798 | 520,046 | 624,593 | ||
| Zero order | 280.919 | 555.142 | 844.7689 | ||
| R2 | 0.468 | 0.475 | 0.4754 | ||
| RSME | 1055 | 1753 | 3170 | ||
| MSE | 1,112,476 | 3,073,787 | 10,049,635 | ||
| Grau | 1.003 | 1.079 | 1.1515 | ||
| 1.137 | 1.149 | 1.1274 | |||
| R2 | 0.998 | 0.998 | 0.9989 | ||
| RSME | 304 | 558 | 630 | ||
| MSE | 92,426 | 310,830 | 396,557 | ||
| Without plant | First order | 0.092 | 0.081 | 0.0832 | |
| R2 | 0.797 | 0.777 | 0.7695 | ||
| RSME | 438 | 209 | 683 | ||
| MSE | 192,075 | 43,871 | 466,723 | ||
| Second order | 0.000020 | 0.000009 | 0.000006 | ||
| R2 | 0.917 | 0.912 | 0.9055 | ||
| RSME | 382 | 1618 | 4580 | ||
| MSE | 145,608 | 2,619,230 | 20,976,788 | ||
| Zero order | 252.831 | 498.344 | 745.9159 | ||
| R2 | 0.422 | 0.444 | 0.4230 | ||
| RSME | 499 | 245 | 798 | ||
| MSE | 248,238 | 60,013 | 636,626 | ||
| Grau | 0.624 | 0.784 | 0.5818 | ||
| 1.279 | 1.295 | 1.3044 | |||
| R2 | 1.000 | 0.999 | 0.9996 | ||
| RSME | 99 | 205 | 221 | ||
| MSE | 9834 | 42,026 | 49,030 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M-Ridha, M.J.; Faeq Ali, M.; Hussein Taly, A.; Abed, K.M.; Mohammed, S.J.; Muhamad, M.H.; Abu Hasan, H. Subsurface Flow Phytoremediation Using Barley Plants for Water Recovery from Kerosene-Contaminated Water: Effect of Kerosene Concentration and Removal Kinetics. Water 2022, 14, 687. https://doi.org/10.3390/w14050687
M-Ridha MJ, Faeq Ali M, Hussein Taly A, Abed KM, Mohammed SJ, Muhamad MH, Abu Hasan H. Subsurface Flow Phytoremediation Using Barley Plants for Water Recovery from Kerosene-Contaminated Water: Effect of Kerosene Concentration and Removal Kinetics. Water. 2022; 14(5):687. https://doi.org/10.3390/w14050687
Chicago/Turabian StyleM-Ridha, Mohanad J., Muna Faeq Ali, Ahmed Hussein Taly, Khalid M. Abed, Sabah J. Mohammed, Mohd Hafizuddin Muhamad, and Hassimi Abu Hasan. 2022. "Subsurface Flow Phytoremediation Using Barley Plants for Water Recovery from Kerosene-Contaminated Water: Effect of Kerosene Concentration and Removal Kinetics" Water 14, no. 5: 687. https://doi.org/10.3390/w14050687