Adsorption and Its Mechanism of Arsenate in Aqueous Solutions by Red Soil
Abstract
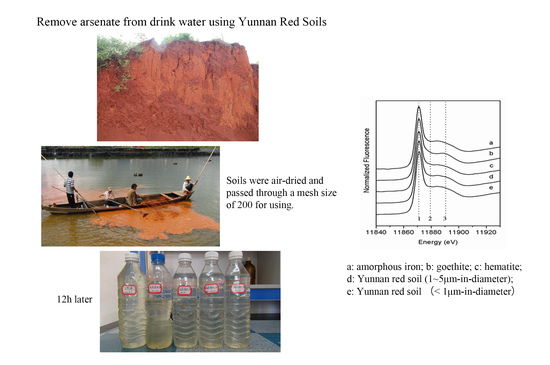
:1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- Sodium arsenate heptahydrate (Na2HAsO4·7H2O) with a purity of 98% was provided by US Alfa Aesar.
- (2)
- A 1% potassium borohydride (KBH4) solution was prepared by dissolving 0.2 g of potassium hydroxide in 100 mL milli Q water, and then 1 g of potassium borohydride was added into the solution and mixed fully.
- (3)
- A 2% hydrochloric acid solution with 10 mL of concentrated hydrochloric acid was diluted to 500 mL with milli Q water. All solutions were prepared using the 2% HCl solution.
- (4)
- Soils and their characterization.
2.2. Equipment
2.3. Batch Adsorption Studies
2.4. Adsorption Mechanism
2.5. Analytical Methods
2.6. Orthogonal Analysis
3. Results and Discussions
3.1. Effect of Soil Types on the Adsorption of Arsenate
3.2. Effect of Soil/Solution Ratios
3.3. Effect of Initial Arsenate Concentration
3.4. Effect of Shaking Speed on the Removal of Arsenate
3.5. Optimization of the Factors Affecting Arsenate Removal by Orthogonal Array
3.6. Adsorption Mechanism of the Red Soils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Ding, T.; Zhang, C. Biosorption and toxicity responses to arsenite (As[III]) in Scenedesmus quadricauda. Chemosphere 2013, 92, 1077–1084. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Jack, C.; Wang, J.; Shraim, A. A global healthy problem caused by arsenic from natural sources. Chemosphere 2003, 52, 1353–1359. [Google Scholar] [CrossRef]
- Yean, S.; Cong, L.; Yavuz, C.; Mayo, J.; Yu, W.; Kan, A.; Colvin, V.; Tomson, M. Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. J. Mater. Res. 2005, 20, 3255–3264. [Google Scholar] [CrossRef]
- Gupta, A.; Yunus, M.; Sankararamakrishnan, N. Zerovalent iron encapsulated chitosan nanospheres—A novel adsorbent for the removal of total inorganic Arsenic from aqueous systems. Chemosphere 2012, 86, 150–155. [Google Scholar] [CrossRef]
- Darrell, K.N. Worldwide occurrences of arsenate in groundwater. Science 2002, 296, 2143–2145. [Google Scholar] [CrossRef]
- Hashim, K.S.; AlKhaddar, R.; Shaw, A.; Kot, P.; Al-Jumeily, D.; Alwash, R.; Aljefery, M.H. Electrocoagulation as an Eco-Friendly River Water Treatment Method. Adv. Water Resour. Eng. Manag. 2020, 39, 219–235. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic(V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef] [PubMed]
- Kanel, S.; Greneche, J.; Choi, H. Arsenic(V) Removal from Groundwater Using Nano Scale Zero-Valent Iron as a Colloidal Reactive Barrier Material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, M.; Charles, U.; Pittman, J. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Ma, Y. Advance in Research on Arsenic Sorption and Its Affecting Factors in Soils. Soils 2008, 40, 351–359. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Z.; Chen, Y.; Liu, L.; Zhang, Z.; Chen, C. Adsorption and Desorption Characteristics of Arsenic on Soils: Kinetics, Equilibrium, and Effect of Fe(OH)3 Colloid, H2SiO3 Colloid and Phosphate. Procedia Environ. Sci. 2013, 18, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Hashim, K.S.; Ewadh, H.M.; Zubaidi, S.L.; Kot, P.; Muradov, M.; Aljefery, M.; Al-Khaddar, R. Phosphate removal from water using bottom ash: Adsortion performance, coexisting anions and modelling studies. Water Sci. Technol. 2021, 83, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Glass, S.; Mantel, T.; Appold, M.; Sen, S.; Usman, M.; Ernst, M.; Filiz, V. Amine-Terminated PAN Membranes as Anion-Adsorber Materials. Chem. Ing. Tech. 2021, 93, 1396–1400. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy Efficient Rapid Removal of Arsenic in an Electrocoagulation Reactor with Hybrid Fe/Al Electrodes: Process Optimization Using CCD and Kinetic Modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance Evaluation of Small Sized Powdered Ferric Hydroxide as Arsenic Adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Agnieszka, A.; Weronika, S.C.; Grzegorz, J. Arsenate Adsorption on Fly Ash, Chitosan and Their Compasites and Its Relations with Surface, Charge and Pore Properties of the Sorbents. Materials 2020, 13, 5381. [Google Scholar] [CrossRef]
- Wang, Z.; He, B.; Pan, X. Levels, trends and risk assessment of arsenic pollution in Yangzonghai Lake, Yunnan Province, China. Sci. China Chem. 2012, 53, 1809–1817. [Google Scholar] [CrossRef]
- Hamayun, M.; Mahmood, T.; Naeem, A. Equilibrium and kinetics studies of arsenate adsorption by FePO4. Chemosphere 2014, 99, 207–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P.; Zhang, J.; Xia, S. Removal and mechanism of Cu (II) and Cd (II) from aqueous single-metal solutions by a novel biosorbent from waste-activated sludge. Environ. Sci. Pollut. Res. 2014, 21, 10823–10829. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, Q.; Gao, S.; Shang, J. Arsenic (III, V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J. Hazard. Mater. 2011, 192, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Sparks, D.; Davis, J. Arsenate adsorption mechanisms at the allophone-water interface. Environ. Sci. Technol. 2005, 39, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Le, G.; Dao, C.; Dang, L.; Nguyen, K.; Nguyen, Q.; Dang, P.; Tran, H.; Duong, Q.; Nguyen, T.; et al. Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv. 2015, 5, 5261–5268. [Google Scholar] [CrossRef]
- Huang, C. Soil Science; China Agriculture Press: Beijing, China, 2000; pp. 169–170. [Google Scholar]
- Zhu, J.; Pigna, M.; Cozzolino, V.; Caporale, A.G.; Violante, A. Sorption of arsenite and arsenate on ferrihydrite: Effect of organic and inorganic ligands. J. Hazard. Mater. 2011, 189, 564–571. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C. Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef]
- Liu, H.L.; Liang, M.; Zhu, Y.; Cai, F.; Zou, H. The Adsorption of Arsenic by Ferric Hydroxide and its Precipitation Mechanism. Acta Sci. Circumstantiae 2009, 29, 1011–1020. [Google Scholar]
- Jia, Y.; Xu, L.; Fang, Z.; Demopoulos, G. Demopoulos. Observation of surface precipitation of arsenate on ferrihydrite. Environ. Sci. Technol. 2006, 40, 3248–3253. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.; Zhang, H.; Haung, J.; Li, Z.; Wei, J. Arsenic forms in various particle-size fractions of red soil-Chemical fractionation and speciation using XANES analysis. Acta Sci. Circumstantiae 2011, 31, 2733–2739. [Google Scholar] [CrossRef]





| Soils | pH | Organic Matter (g/kg) | Soil Physical Sand Content (1~0.01 mm) % | Soil Physical Clay Content (<0.01 mm) % | Fe2O3/% | Al2O3/% |
|---|---|---|---|---|---|---|
| Yunnan red soil 1 | 6.59 | 9.3 | 70.7 | 29.3 | 14.04 | 28.86 |
| Yunnan red soil 2 | 6.47 | 9.2 | 57.3 | 42.7 | 10.05 | 24.48 |
| Yunnan red soil 3 | 5.93 | 10.2 | 60.8 | 39.2 | 12.59 | 26.70 |
| Yunnan red soil 4 | 5.88 | 11.9 | 73.3 | 26.7 | 12.86 | 26.51 |
| Jiangxi red soil | 6.24 | 17.0 | 87.9 | 12.1 | 8.91 | 18.28 |
| Guangdong latosol | 6.55 | 15.1 | 59.2 | 40.8 | 8.29 | 18.56 |
| Initial Concentration (mg/L) | Yunnan Red Soil 1 | Yunnan Red Soil 4 | ||||
|---|---|---|---|---|---|---|
| Removal Rate (%) | RSD (%) | Adsorption Capacity (μg/g) | Removal Rate (%) | RSD (%) | Adsorption Capacity (μg/g) | |
| 0.05 | 95.1 | 4.0 | 158.5 | 95.5 | 5.7 | 159.1 |
| 0.1 | 92.2 | 5.6 | 307.3 | 93.2 | 8.1 | 310.7 |
| 0.2 | 79.5 | 5.4 | 529.8 | 82.5 | 5.2 | 550.3 |
| 0.5 | 58.8 | 3.6 | 979.2 | 59.3 | 0.7 | 987.7 |
| 1.0 | 41.6 | 0.9 | 1387.3 | 42.8 | 1.8 | 1427.7 |
| 2.0 | 20.8 | 3.7 | 1389.2 | 24.6 | 0.1 | 1637.1 |
| No. | A ※. Soil Type | B ※. Soil/Solution Ratio (g/mL) | C ※. Contact Time (min) | D ※. Shaking Speed (rpm) | Removal Rate (%) |
|---|---|---|---|---|---|
| 1 | 1 (Yunnan red soil 1) | 1 (0.025/100) | 1 (15) | 1 (200) | 64.1 |
| 2 | 1 | 2 (0.030/100) | 2 (30) | 2 (225) | 76.5 |
| 3 | 1 | 3 (0.035/100) | 3 (45) | 3 (250) | 84.8 |
| 4 | 2 (Yunnan red soil 2) | 1 | 2 | 3 | 47.0 |
| 5 | 2 | 2 | 3 | 1 | 55.8 |
| 6 | 2 | 3 | 1 | 2 | 54.2 |
| 7 | 3 (Yunnan red soil 4) | 1 | 3 | 2 | 70.7 |
| 8 | 3 | 2 | 1 | 3 | 69.6 |
| 9 | 3 | 3 | 2 | 1 | 78.8 |
| k1 | 225.4 | 181.8 | 187.9 | 198.7 | |
| k2 | 157.0 | 201.9 | 202.3 | 201.4 | |
| k3 | 219.1 | 217.8 | 211.3 | 201.4 | |
| K1 | 75.1 | 60.6 | 62.6 | 66.2 | |
| K2 | 52.3 | 67.3 | 67.4 | 67.1 | |
| K3 | 73.0 | 72.6 | 70.4 | 67.1 | |
| R (max-min) | 22.8 | 12.0 | 7.8 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Shi, L.; Gu, W.; Wu, W. Adsorption and Its Mechanism of Arsenate in Aqueous Solutions by Red Soil. Water 2022, 14, 579. https://doi.org/10.3390/w14040579
Guo M, Shi L, Gu W, Wu W. Adsorption and Its Mechanism of Arsenate in Aqueous Solutions by Red Soil. Water. 2022; 14(4):579. https://doi.org/10.3390/w14040579
Chicago/Turabian StyleGuo, Min, Lili Shi, Wen Gu, and Wenzhu Wu. 2022. "Adsorption and Its Mechanism of Arsenate in Aqueous Solutions by Red Soil" Water 14, no. 4: 579. https://doi.org/10.3390/w14040579