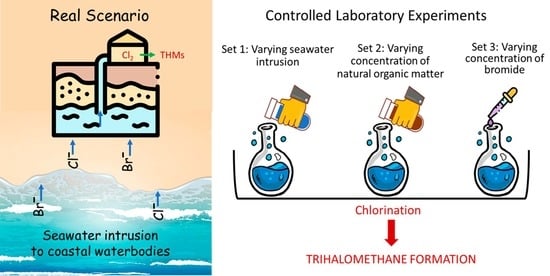
Effect of Seawater Intrusion on the Formation of Chlorinated and Brominated Trihalomethanes in Coastal Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Methods
2.2. Experiments
- Set 1: Varying extent of seawater intrusion
- Set 2: Varying natural organic matter concentrations
- Set 3: Varying bromide concentrations
2.3. Bromine Substitution Factor
3. Results
3.1. Effect of Seawater Intrusion on Raw Water Characteristics
3.2. Chlorine Demand
3.3. Trihalomethane Formation and Speciation
3.4. Effect of Natural Organic Matter
3.5. Effect of Chlorine Demand, Chloride, and Bromide Concentrations
3.6. Bromine Substitution Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, M.S.; Abd-Elhamid, H.F.; Javadi, A.A.; Sherif, M.M. Management of Seawater Intrusion in Coastal Aquifers: A Review. Water 2019, 11, 2467. [Google Scholar] [CrossRef] [Green Version]
- Alfarrah, N.; Walraevens, K. Groundwater Overexploitation and Seawater Intrusion in Coastal Areas of Arid and Semi-Arid Regions. Water 2018, 10, 143. [Google Scholar] [CrossRef] [Green Version]
- Mastrocicco, M.; Busico, G.; Colombani, N.; Vigliotti, M.; Ruberti, D. Modelling Actual and Future Seawater Intrusion in the Variconi Coastal Wetland (Italy) Due to Climate and Landscape Changes. Water 2019, 11, 1502. [Google Scholar] [CrossRef] [Green Version]
- Luoma, S.; Okkonen, J. Impacts of Future Climate Change and Baltic Sea Level Rise on Groundwater Recharge, Groundwater Levels, and Surface Leakage in the Hanko Aquifer in Southern Finland. Water 2014, 6, 3671–3700. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, F.J.; Rey, A.; Gimeno, O. The Role of Catalytic Ozonation Processes on the Elimination of DBPs and Their Precursors in Drinking Water Treatment. Catalysts 2021, 11, 521. [Google Scholar] [CrossRef]
- Stein, S.; Sivan, O.; Yechieli, Y.; Kasher, R.; Nir, O. An Advantage for Desalination of Coastal Saline Groundwater over Seawater in View of Boron Removal Requirements. Environ. Sci. Water Res. Technol. 2021, 7, 2241–2254. [Google Scholar] [CrossRef]
- Goel, S. Water and Wastewater Engineering, 1st ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; ISBN 978-1-316-63903-0. [Google Scholar]
- Deborde, M.; von Gunten, U. Reactions of Chlorine with Inorganic and Organic Compounds during Water Treatment—Kinetics and Mechanisms: A Critical Review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef]
- Wong, G.T.F.; Davidson, J.A. The Fate of Chlorine in Sea-Water. Water Res. 1977, 11, 971–978. [Google Scholar] [CrossRef]
- Chowdhury, S. Effects of Seawater Intrusion on the Formation of Disinfection Byproducts in Drinking Water. Sci. Total Environ. 2022, 827, 154398. [Google Scholar] [CrossRef]
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental Impacts of the Widespread Use of Chlorine-Based Disinfectants during the COVID-19 Pandemic. Environ. Sci. Pollut. Res. 2022, 1–19. [Google Scholar] [CrossRef]
- Ayyandurai, R.; Venkateswaran, S.; Karunanidhi, D. Hydrogeochemical Assessment of Groundwater Quality and Suitability for Irrigation in the Coastal Part of Cuddalore District, Tamil Nadu, India. Mar. Pollut. Bull. 2022, 174, 113258. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, J.; Sekar, S.; Roy, P.D.; Senapathi, V.; Chung, S.Y.; Perumal, M.; Nath, A.V. Groundwater Pollution Index (GPI) and GIS-Based Appraisal of Groundwater Quality for Drinking and Irrigation in Coastal Aquifers of Tiruchendur, South India. Environ. Sci. Pollut. Res. 2021, 28, 29056–29074. [Google Scholar] [CrossRef] [PubMed]
- Ged, E.C.; Boyer, T.H. Effect of Seawater Intrusion on Formation of Bromine-Containing Trihalomethanes and Haloacetic Acids during Chlorination. Desalination 2014, 345, 85–93. [Google Scholar] [CrossRef]
- Szczuka, A.; Parker, K.M.; Harvey, C.; Hayes, E.; Vengosh, A.; Mitch, W.A. Regulated and Unregulated Halogenated Disinfection Byproduct Formation from Chlorination of Saline Groundwater. Water Res. 2017, 122, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Young, R.T.; Deem, S.; Leslie, J.C.; Salo-Zieman, V.; He, H.; Dodd, M.C. Drivers of Disinfection Byproduct Formation and Speciation in Small, Chlorinated Coastal Groundwater Systems: Relative Roles of Bromide and Organic Matter, and the Need for Improved Source Water Characterization and Monitoring. Environ. Sci. Water Res. Technol. 2020, 6, 3361–3379. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, G.; Lu, X. Characteristics of DOM and Removal of DBPs Precursors across O3-BAC Integrated Treatment for the Micro-Polluted Raw Water of the Huangpu River. Water 2013, 5, 1472–1486. [Google Scholar] [CrossRef] [Green Version]
- Dubowski, Y.; Greenberg-Eitan, R.; Rebhun, M. Removal of Trihalomethane Precursors by Nanofiltration in Low-SUVA Drinking Water. Water 2018, 10, 1370. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Yu, Y.; Huang, X. Comparison of Organic Matter Properties and Disinfection By-Product Formation between the Typical Groundwater and Surface Water. Water 2022, 14, 1418. [Google Scholar] [CrossRef]
- APHA; WEF; AWWA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- USEPA. EPA Method 501.1, The Analysis of Trihalomethanes in Drinking Water by He Purge and Trap Method; Genium Publishing Corporation: Schenectady, NY, USA, 1979.
- ASTM Standard Practice for Preparation of Substitute Ocean Water. 2021. Available online: https://www.astm.org/d1141-98r21.html (accessed on 25 September 2022).
- Boyer, T.H.; Singer, P.C. Bench-Scale Testing of a Magnetic Ion Exchange Resin for Removal of Disinfection by-Product Precursors. Water Res. 2005, 39, 1265–1276. [Google Scholar] [CrossRef]
- Summers, R.S.; Hooper, S.M.; Shukairy, H.M.; Solarik, G.; Owen, D. Assessing DBP Yield: Uniform Formation Conditions. J. —Am. Water Work. Assoc. 1996, 88, 80–93. [Google Scholar] [CrossRef]
- Parveen, N.; Ranjan, V.P.; Chowdhury, S.; Goel, S. Occurrence and Potential Health Risks Due to Trihalomethanes and Microplastics in Bottled Water. Environ. Eng. Sci. 2022, 39, 523–534. [Google Scholar] [CrossRef]
- Font-Ribera, L.; Kogevinas, M.; Schmalz, C.; Zwiener, C.; Marco, E.; Grimalt, J.O.; Liu, J.; Zhang, X.; Mitch, W.; Critelli, R.; et al. Environmental and Personal Determinants of the Uptake of Disinfection By-Products during Swimming. Environ. Res. 2016, 149, 206–215. [Google Scholar] [CrossRef] [PubMed]
- BIS. Indian Standard 10500 Drinking Water—Specifications (Second Revision); Bureau of Indian Standards: New Delhi, India, 2012; p. 16.
- Stefán, D.; Balogh, J.; Záray, G.; Vargha, M. Comparison of Disinfection By-Product Formation and Distribution during Breakpoint Chlorination and Chlorine-Based Disinfection in Drinking Water. Water 2022, 14, 1372. [Google Scholar] [CrossRef]
- Padhi, R.K. Carbonaceous DBP (THMs and HAAs) Formation during Cl2 and ClO2 Treatment of Aqueous Soluble Fractions of Soil Derived Natural Organic Matter. Environ. Sci. Water Res. Technol. 2022, 8, 597–606. [Google Scholar] [CrossRef]
- Sharma, N.; Mohapatra, S.; Padhye, L.P.; Mukherji, S. Role of Precursors in the Formation of Trihalomethanes during Chlorination of Drinking Water and Wastewater Effluents from a Metropolitan Region in Western India. J. Water Process Eng. 2021, 40, 101928. [Google Scholar] [CrossRef]
- Golea, D.M.; Upton, A.; Jarvis, P.; Moore, G.; Sutherland, S.; Parsons, S.A.; Judd, S.J. THM and HAA Formation from NOM in Raw and Treated Surface Waters. Water Res. 2017, 112, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyung Kim, M.; Yu, M.J. Characterization of NOM in the Han River and Evaluation of Treatability Using UF–NF Membrane. Environ. Res. 2005, 97, 116–123. [Google Scholar] [CrossRef]
- Ding, G.; Zhang, X.; Yang, M.; Pan, Y. Formation of New Brominated Disinfection Byproducts during Chlorination of Saline Sewage Effluents. Water Res. 2013, 47, 2710–2718. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Richardson, S.D. Formation of Iodinated Disinfection Byproducts (I-DBPs) in Drinking Water: Emerging Concerns and Current Issues. Acc. Chem. Res. 2019, 52, 896–905. [Google Scholar] [CrossRef]
- Bernstein, A.; Studny, R.; Shyntychea, V.; Kurtzman, D.; Ganot, Y.; Katz, Y.; Asfaw, B.A.; Sakaguchi-Söder, K.; Schüth, C.; Siebner, H. Low Trihalomethane Formation during Managed Aquifer Recharge with Chlorinated Desalinated Water. Water 2020, 12, 711. [Google Scholar] [CrossRef]
- Goyetche, T.; Luquot, L.; Carrera, J.; Martínez-Pérez, L.; Folch, A. Identification and Quantification of Chemical Reactions in a Coastal Aquifer to Assess Submarine Groundwater Discharge Composition. Sci. Total Environ. 2022, 838, 155978. [Google Scholar] [CrossRef] [PubMed]
- Heeb, M.B.; Criquet, J.; Zimmermann-Steffens, S.G.; von Gunten, U. Oxidative Treatment of Bromide-Containing Waters: Formation of Bromine and Its Reactions with Inorganic and Organic Compounds—A Critical Review. Water Res. 2014, 48, 15–42. [Google Scholar] [CrossRef] [PubMed]




| Reference | General Experimental Procedure | Type of Seawater Used | Chlorine Dosage | Was the Effect of NOM on THM Formation Evaluated |
|---|---|---|---|---|
| [14] | Samples: One groundwater and one seawater sample from Florida, USA. SWI simulation: Groundwater sample diluted to achieve a constant DOC of approximately 1.4 mg/L. Seawater added to the diluted groundwater at 0–2% by volume. | Real seawater | As per UFC 1 | No |
| [15] | Sample: 24 SWI-affected groundwater samples from North Carolina, USA. Chlorinated in laboratory. No SWI simulation. | - | To yield a free residual chlorine of 1 mg/L after 24 h without pH adjustment. | No |
| [16] | Samples: Different coastal groundwater systems of Washington, USA. Filtered, buffered at pH 7, and chlorinated. No SWI simulation. | - | To yield a free residual chlorine of 3–5 mg/L after 7 days incubation at 25 °C. | Relative role of bromide to DOC was analysed without controlled NOM experiments |
| [10] | Sample: Groundwater and seawater samples from Dhahran, Saudi Arabia. SWI simulation: Groundwater diluted to yield a DOC similar to finished water. SWI simulation by mixing diluted groundwater and 0–2% seawater by volume. | Real seawater | 2 mg/L of chlorine for a reaction period of 8 h | No |
| Current study | Three sets of experiments for varying degrees of SWI, NOM, and Br concentrations (Section 2.2) | Real seawater and synthetic seawater | As per UFC, samples were analysed after 24 h and 48 h | Yes. Different concentrations of NOM, chloride, and bromide were added to the reaction mixtures to evaluate the effects on THM formation |
| Type of Seawater | Seawater Intrusion (% Volume) | pH | DOC (mg/L) | Chloride (mg/L) | Chloride (mM) | Bromide (mg/L) | Bromide (mM) | Cl/Br Ratio (Mass Basis) | Cl/Br Ratio (Molar Basis) |
|---|---|---|---|---|---|---|---|---|---|
| SSW | 0% | 7.1 | 1.4 | 2.42 | 0.068 | 0 | 0 | - | - |
| 0.25% | 7.45 | 1.5 | 55.35 | 1.56 | 0.19 | 0.002 | 290.1 | 653.8 | |
| 0.5% | 7.52 | 1.6 | 118.2 | 3.33 | 0.40 | 0.005 | 290.1 | 653.9 | |
| 1% | 7.59 | 1.6 | 220.1 | 6.20 | 0.75 | 0.010 | 291.0 | 656.0 | |
| 2% | 7.74 | 1.7 | 400.7 | 11.30 | 1.39 | 0.017 | 288.7 | 650.6 | |
| 3% | 7.98 | 1.7 | 635.9 | 17.93 | 2.19 | 0.027 | 290.9 | 655.7 | |
| RSW | 0% | 7.1 | 1.4 | 2.42 | 0.068 | 0 | 0 | - | - |
| 0.25% | 7.65 | 1.6 | 87.1 | 2.45 | 0.52 | 0.006 | 164.6 | 371.1 | |
| 0.5% | 7.80 | 1.6 | 175.1 | 4.94 | 1.05 | 0.013 | 162.7 | 373.2 | |
| 1% | 7.95 | 1.8 | 352.4 | 9.93 | 2.12 | 0.026 | 158.1 | 373.2 | |
| 2% | 8.11 | 1.8 | 698.4 | 19.70 | 4.20 | 0.052 | 151.8 | 373.9 | |
| 3% | 8.20 | 1.9 | 1045.2 | 29.48 | 6.32 | 0.079 | 142.0 | 372.3 | |
| RSW | - | 33,925.8 | 956.9 | 204.2 | 2.55 | 166.13 | 374.5 | ||
| Reference seawater 1 | - | 19,862 | 560.23 | 69.05 | 0.864 | 287.64 | 648.3 |
| THM | Direction of Change | Reaction Period = 24h | Reaction Period = 48h | ||
|---|---|---|---|---|---|
| Change in THM Concentration Per Unit Change in Cl/Br Ratio (µM of THM Per Cl/Br) | p Value/R2 | Change in THM Concentration Per Unit Change in Cl/Br Ratio (µM of THM Per Cl/Br) | p Value/R2 | ||
| TCM | Increase | 2.44 × 10−4 | 0.01/0.91 | 4.13 × 10−4 | 0.02/0.84 |
| BDCM | Decrease | 2.02 × 10−4 | 0.006/0.94 | 1.08 × 10−4 | 0.007/0.93 |
| DBCM | Decrease | 5.50 × 10−4 | 0.029/0.84 | insignificant | p > 0.05 |
| TBM | Decrease | 9.27 × 10−4 | 0.003/0.96 | 1 × 10−3 | 0.004/0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveen, N.; Goel, S. Effect of Seawater Intrusion on the Formation of Chlorinated and Brominated Trihalomethanes in Coastal Groundwater. Water 2022, 14, 3579. https://doi.org/10.3390/w14213579
Parveen N, Goel S. Effect of Seawater Intrusion on the Formation of Chlorinated and Brominated Trihalomethanes in Coastal Groundwater. Water. 2022; 14(21):3579. https://doi.org/10.3390/w14213579
Chicago/Turabian StyleParveen, Naseeba, and Sudha Goel. 2022. "Effect of Seawater Intrusion on the Formation of Chlorinated and Brominated Trihalomethanes in Coastal Groundwater" Water 14, no. 21: 3579. https://doi.org/10.3390/w14213579