Application of Conductive Concrete as a Microbial Fuel Cell to Control H2S Emission for Mitigating Sewer Corrosion
Abstract
:1. Introduction
2. Materials and Methods
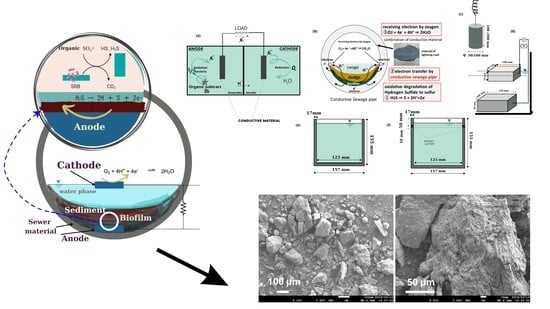
2.1. Models and Materials
2.2. Electrical Resistivity
2.3. Sample Preparation
2.4. Experimental Procedure and Mechanism Assessment
2.4.1. Adsorption Assessment of Conductive Material regarding H2S
2.4.2. Voltage Measurement Experiments Using Plate-Shaped Specimens
2.4.3. Hydrogen Sulfide Suppression Experiment
2.4.4. Hydrogen Sulfide Suppression with Epoxy-Layer Cup-Shaped Specimen
2.5. Analysis Instructions
3. Results
3.1. Measurement of Electrical Resistivity
3.2. Results of Adsorption Experiment with Hydrogen Sulfide
3.3. Voltage Measurement Experiments Using Plate-Shaped Specimens
3.4. Hydrogen Sulfide Suppression Experiment Using Cup-Shaped Specimen
3.5. Hydrogen Sulfide Suppression Experiments Using Cup-Shaped Specimens of Sun Earth Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firer, D.; Friedler, E.; Lahav, O. Control of sulfide in sewer systems by dosage of iron salts: Comparison between theoretical and experimental results, and practical implications. Sci. Total Environ. 2008, 392, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.; Mohanakrishnan, J.; Sharma, K.R.; Meyer, R.L.; Keller, J.; Yuan, Z. Evaluation of oxygen injection as a means of controlling sulfide production in a sewer system. Water Res. 2008, 42, 4549–4561. [Google Scholar] [CrossRef]
- Gutierrez, O.; Sudarjanto, G.; Ren, G.; Ganigué, R.; Jiang, G.; Yuan, Z. Assessment of pH shock as a method for controlling sulfide and methane formation in pressure main sewer systems. Water Res. 2014, 48, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Gutierrez, O.; Sharma, K.R.; Keller, J.; Yuan, Z. Optimization of intermittent, simultaneous dosage of nitrite and hydrochloric acid to control sulfide and methane productions in sewers. Water Res. 2011, 45, 6163–6172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Keating, A.; Corrie, S.; O’halloran, K.; Nguyen, L.; Yuan, Z. Dosing free nitrous acid for sulfide control in sewers: Results of field trials in Australia. Water Res. 2013, 47, 4331–4339. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, G.; Bond, P.L.; Keller, J.; Yuan, Z. A novel and simple treatment for control of sulfide induced sewer concrete corrosion using free nitrous acid. Water Res. 2015, 70, 279–287. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G. Mitigation of Microbially Influenced Corrosion of Concrete Sewers Using Nitrite. In Biotechnological Innovations for Environmental Bioremediation; Arora, S., Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer: Singapore, 2022; pp. 119–135. ISBN 978-981-16-9001-3. [Google Scholar]
- Pikaar, I.; Flugen, M.; Lin, H.W.; Salehin, S.; Li, J.; Donose, B.C.; Dennis, P.G.; Bethke, L.; Johnson, I.; Rabaey, K.; et al. Full-scale investigation of in-situ iron and alkalinity generation for efficient sulfide control. Water Res. 2019, 167, 115032. [Google Scholar] [CrossRef]
- Nemati, M.; Jenneman, G.E.; Voordouw, G. Mechanistic study of microbial control of hydrogen sulfide production in oil reservoirs. Biotechnol. Bioeng. 2001, 74, 424–434. [Google Scholar] [CrossRef]
- Lee, E.K.; Jung, K.D.; Joo, O.S.; Shul, Y.G. Liquid-phase oxidation of hydrogen sulfide to sulfur over CuO/MgO catalyst. React. Kinet. Catal. Lett. 2005, 87, 115–120. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chang, Y.T.; Chen, H.J. A method for controlling hydrogen sulfide in water by adding solid phase oxygen. Bioresour. Technol. 2007, 98, 478–483. [Google Scholar] [CrossRef]
- Aguilar, L.; Zha, S.; Cheng, Z.; Winnick, J.; Liu, M. A solid oxide fuel cell operating on hydrogen sulfide (H2S) and sulfur-containing fuels. J. Power Sources 2004, 135, 17–24. [Google Scholar] [CrossRef]
- Marcus, A.K.; Torres, C.I.; Rittmann, B.E. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 2007, 98, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Imai, T.; Ukita, M.; Sekine, M.; Higuchi, T. Triggering forces for anaerobic granulation in UASB reactors. Process. Biochem. 2006, 41, 36–43. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochizuki, T.; Kubota, M.; Matsuda, H.; D’Elia Camacho, L.F. Adsorption behaviors of ammonia and hydrogen sulfide on activated carbon prepared from petroleum coke by KOH chemical activation. Fuel Process. Technol. 2016, 144, 164–169. [Google Scholar] [CrossRef]
- Shi, L.; Yang, K.; Zhao, Q.; Wang, H.; Cui, Q. Characterization and Mechanisms of H2S and SO2 Adsorption by Activated Carbon. Energy Fuels 2015, 29, 6678–6685. [Google Scholar] [CrossRef]
- Katoh, H.; Kuniyoshi, I.; Hirai, M.; Shoda, M. Studies of the oxidation mechanism of sulphur-containing gases on wet activated carbon fibre. Appl. Catal. B Environ. 1995, 6, 255–262. [Google Scholar] [CrossRef]
- Yan, R.; Liang, D.T.; Tsen, L.; Tay, J.H. Kinetics and mechanisms of H2S adsorption by alkaline activated carbon. Environ. Sci. Technol. 2002, 36, 4460–4466. [Google Scholar] [CrossRef]
- Rabaey, K.; Van De Sompel, K.; Maignien, L.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Pham, H.T.; Vermeulen, J.; Verhaege, M.; et al. Microbial fuel cells for sulfide removal. Environ. Sci. Technol. 2006, 40, 5218–5224. [Google Scholar] [CrossRef]
- Liu, S.H.; Lai, Y.C.; Lin, C.W. Enhancement of power generation by microbial fuel cells in treating toluene-contaminated groundwater: Developments of composite anodes with various compositions. Appl. Energy 2019, 233–234, 922–929. [Google Scholar] [CrossRef]
- González, T.; Ureta-Zañartu, M.S.; Marco, J.F.; Vidal, G. Effect of Zeolite-Fe on graphite anode in electroactive biofilm development for application in microbial fuel cells. Appl. Surf. Sci. 2019, 467–468, 851–859. [Google Scholar] [CrossRef]









| Chemicals | NaHCO3 | K2HPO4 | Yeast Extract | Glucose | (NH4)2HPO4 | KCL | NH4Cl | FeCl3 6H2O | MgCl2 6H2O | MgSO4 7H2O | CoCl2 6H2O | CaCl2 6H2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 2 | 2 | 0.02 | 2 | 0.7 | 0.75 | 0.85 | 0.42 | 0.81 | 0.25 | 0.18 | 0.15 |
| Mixture of Sludge | Anaerobic Sludge (mg/L) | Aerobic Sludge (mg/L) | ||
|---|---|---|---|---|
| SS | VSS | SS | VSS | |
| Mix 1 | 10,200 | 8600 | 1560 | 1200 |
| Mix 2 | 31,800 | 14,200 | 2980 | 2300 |
| Mix 3 | 11,800 | 8600 | 5700 | 4700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, T.; Vo, H.T.; Fukushima, M.; Suzuki, T.; Sakuma, H.; Hitomi, T.; Hung, Y.-T. Application of Conductive Concrete as a Microbial Fuel Cell to Control H2S Emission for Mitigating Sewer Corrosion. Water 2022, 14, 3454. https://doi.org/10.3390/w14213454
Imai T, Vo HT, Fukushima M, Suzuki T, Sakuma H, Hitomi T, Hung Y-T. Application of Conductive Concrete as a Microbial Fuel Cell to Control H2S Emission for Mitigating Sewer Corrosion. Water. 2022; 14(21):3454. https://doi.org/10.3390/w14213454
Chicago/Turabian StyleImai, Tsuyoshi, Huy Thanh Vo, Masato Fukushima, Tasuma Suzuki, Hiraku Sakuma, Takashi Hitomi, and Yung-Tse Hung. 2022. "Application of Conductive Concrete as a Microbial Fuel Cell to Control H2S Emission for Mitigating Sewer Corrosion" Water 14, no. 21: 3454. https://doi.org/10.3390/w14213454