Catalytic Ozonation of Atrazine Enhanced by Mesoporous CeO2: Morphology, Performance and Intermediates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CeO2
2.3. Experimental Procedure
2.4. Characterization
3. Results and Discussion
3.1. Characterization of CeO2
3.2. Catalytic Ozonation of Atrazine
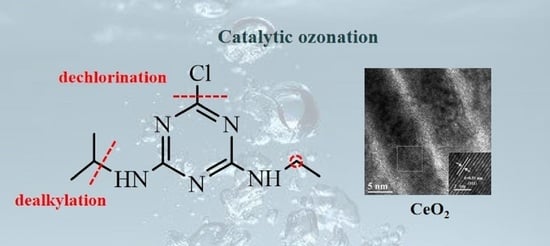
3.3. Transformation Products and Proposed Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salazar-Ledesma, M.; Prado, B.; Zamora, O.; Siebe, C. Mobility of atrazine in soils of a wastewater irrigated maize field. Agric. Ecosyst. Environ. 2018, 255, 73–83. [Google Scholar] [CrossRef]
- Urseler, N.; Bachetti, R.; Biolé, F.; Morgante, V.; Morgante, C. Atrazine pollution in groundwater and raw bovine milk: Water quality, bioaccumulation and human risk assessment. Sci. Total Environ. 2022, 852, 158498. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, S.-S.; Feng, Z.; Fu, H.; Wang, M.; Wang, P.; Liu, W.; Wang, C.-C. High-efficient peroxymonosulfate activation for rapid atrazine degradation by FeSx@MoS2 derived from MIL-88A(Fe). J. Hazard. Mater. 2022, 440, 129723. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cong, J.; Sun, Y.; Li, G.; Zhou, Z.; Qian, C.; Liu, F. Determination of Endocrine Disrupting Chemicals in Surface Water and Industrial Wastewater from Beijing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Navarra, W.; Sacco, O.; Daniel, C.; Venditto, V.; Vaiano, V.; Vignati, D.A.L.; Bojic, C.; Libralato, G.; Lofrano, G.; Carotenuto, M. Photocatalytic degradation of atrazine by an N-doped TiO2/polymer composite: Catalytic efficiency and toxicity evaluation. J. Environ. Chem. Eng. 2022, 10, 108167. [Google Scholar] [CrossRef]
- Yeom, Y.; Han, J.; Zhang, X.; Shang, C.; Zhang, T.; Li, X.; Duan, X.; Dionysiou, D.D. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants. Chem. Eng. J. 2021, 424, 130053. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Sattar, M.A.; Jahirul, M.I. Phenol degradation of waste and stormwater on a flat plate photocatalytic reactor with TiO2 on glass slide: An experimental and modelling investigation. J. Water Process Eng. 2022, 47, 102769. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Rostami, S.; Jafari, S.; Moeini, Z.; Jaskulak, M.; Keshtgar, L.; Badeenezhad, A.; Azhdarpoor, A.; Rostami, M.; Zorena, K.; Dehghani, M. Current methods and technologies for degradation of atrazine in contaminated soil and water: A review. Environ. Technol. Innov. 2021, 24, 102019. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Li, H.; Zhou, B. Degradation of atrazine by catalytic ozonation in the presence of iron scraps: Performance, transformation pathway, and acute toxicity. J. Environ. Sci. Health B 2019, 54, 432–440. [Google Scholar] [CrossRef]
- Ye, G.; Luo, P.; Zhao, Y.; Qiu, G.; Hu, Y.; Preis, S.; Wei, C. Three-dimensional Co/Ni bimetallic organic frameworks for high-efficient catalytic ozonation of atrazine: Mechanism, effect parameters, and degradation pathways analysis. Chemosphere 2020, 253, 126767. [Google Scholar] [CrossRef]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on discharge Plasma for water treatment: Mechanism, reactor geometries, active species and combined processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Chen, Z.; Huang, X.; Wang, X.; Zhang, X.; Wen, X. Novel catalytic ceramic membranes anchored with MnMe oxide and their catalytic ozonation performance towards atrazine degradation. J. Membr. Sci. 2022, 648, 120362. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Nie, Y.; Yang, M.; Qu, J. Mechanism of Catalytic Ozonation in Fe2O3/Al2O3@SBA-15 Aqueous Suspension for Destruction of Ibuprofen. Environ. Sci. Technol. 2015, 49, 1690–1697. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Yan, P.; Chen, Z. Interface Mechanisms of Catalytic Ozonation with Amorphous Iron Silicate for Removal of 4-Chloronitrobenzene in Aqueous Solution. Environ. Sci. Technol. 2018, 52, 1429–1434. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, T.; Liu, S.; Zhang, G.C.; Huo, K. Catalytic ozonation of phenol enhanced by mesoporous MnO2 prepared through nanocasting method with SBA-15 as template. J. Environ. Chem. Eng. 2020, 8, 103967. [Google Scholar] [CrossRef]
- Matheswaran, M.; Balaji, S.; Chung, S.J.; Moon, I.S. Studies on cerium oxidation in catalytic ozonation process: A novel approach for organic mineralization. Catal. Commun. 2007, 8, 1497–1501. [Google Scholar] [CrossRef]
- Afzal, S.; Quan, X.; Lu, S. Catalytic performance and an insight into the mechanism of CeO2 nanocrystals with different exposed facets in catalytic ozonation of p-nitrophenol. Appl. Catal. B Environ. 2019, 248, 526–537. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Fan, S.; Song, J.; Xia, Y.; Dai, Q. Catalytic ozonation of thymol with a novel CoCe-MMO catalyst: Kinetics and mechanism. Environ. Technol. Innov. 2021, 24, 101881. [Google Scholar] [CrossRef]
- Zuo, X.; Ma, S.; Wu, Q.; Xiong, J.; He, J.; Ma, C.; Chen, Z. Nanometer CeO2 doped high silica ZSM-5 heterogeneous catalytic ozonation of sulfamethoxazole in water. J. Hazard. Mater. 2021, 411, 125072. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Cui, Y.; Wang, S.; Liu, B.; Liu, C. Enhanced photocatalytic activation of peroxymonosulfate by CeO2 incorporated ZnCo–layered double hydroxide toward organic pollutants removal. Sep. Purif. Technol. 2021, 263, 118413. [Google Scholar] [CrossRef]
- Amri, A.; Duan, X.; Yin, C.-Y.; Jiang, Z.-T.; Rahman, M.M.; Pryor, T. Solar absorptance of copper–cobalt oxide thin film coatings with nano-size, grain-like morphology: Optimization and synchrotron radiation XPS studies. Appl. Surf. Sci. 2013, 275, 127–135. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M.; Gautier, J.L.; Ríos, E.; Berry, F.J. Characterization of the Nickel Cobaltite, NiCo2O4, Prepared by Several Methods: An XRD, XANES, EXAFS, and XPS Study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Gao, J.; Tang, L.; Shen, Z.; Dong, Y.; Wang, Z.; Lyu, J.; Li, J.; Yu, H.-Q. Coupling of SiC and CeO2 nanosheets to enhance solar energy utilization and optimize catalytic ozonation. Appl. Catal. B Environ. 2022, 317, 121697. [Google Scholar] [CrossRef]
- Yuan, X.; Yan, X.; Xu, H.; Li, D.; Sun, L.; Cao, G.; Xia, D. Enhanced ozonation degradation of atrazine in the presence of nano-ZnO: Performance, kinetics and effects. J. Environ. Sci. 2017, 61, 3–13. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, B.; Yu, Y.; Bu, L.; Deng, J.; Zhou, S. Heterogeneous catalysis of ozone using ordered mesoporous Fe3O4 for degradation of atrazine. Chem. Eng. J. 2017, 328, 527–535. [Google Scholar] [CrossRef]
- Qu, Z.; Xu, X.; Ren, H.; Sun, T.; Huang, L.; Gao, Z. Effective mineralization of p-nitrophenol in water by heterogeneous catalytic ozonation using Ce-loaded sepiolite catalyst. J. Environ. Chem. Eng. 2022, 10, 108185. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, H.; Peng, P.; Bo, H. Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J. Hazard. Mater. 2014, 279, 444–451. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Model applications and mechanism study on the degradation of atrazine by Fenton’s system. J. Hazard. Mater. 2005, 118, 227–237. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Ma, J.; Lu, X.; Qi, J.; Song, S. Strong promoted catalytic ozonation of atrazine at low temperature using tourmaline as catalyst: Influencing factors, reaction mechanisms and pathways. Chem. Eng. J. 2018, 354, 113–125. [Google Scholar] [CrossRef]
- Qiu, X.H.; Su, X.Y.; Li, X.J.; Li, N. Preparation of CeO2 with Different Morphologies and Its Application to Catalytic Ozonation of Aqueous Lemon Yellow Solutions. Adv. Mater. Res. 2014, 997, 3–8. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhuang, T.; Liu, S.; Sun, S.; Wang, Y.; Liu, X.; Wang, J.; Liu, R. Catalytic Ozonation of Atrazine Enhanced by Mesoporous CeO2: Morphology, Performance and Intermediates. Water 2022, 14, 3431. https://doi.org/10.3390/w14213431
Zhang J, Zhuang T, Liu S, Sun S, Wang Y, Liu X, Wang J, Liu R. Catalytic Ozonation of Atrazine Enhanced by Mesoporous CeO2: Morphology, Performance and Intermediates. Water. 2022; 14(21):3431. https://doi.org/10.3390/w14213431
Chicago/Turabian StyleZhang, Jianlin, Tao Zhuang, Shanjun Liu, Shan Sun, Yongxin Wang, Xinyu Liu, Jin Wang, and Rutao Liu. 2022. "Catalytic Ozonation of Atrazine Enhanced by Mesoporous CeO2: Morphology, Performance and Intermediates" Water 14, no. 21: 3431. https://doi.org/10.3390/w14213431



_Dionysiou.jpg)