The Occurrence and Role of Tetrasphaera in Enhanced Biological Phosphorus Removal Systems
Abstract
:1. Introduction
2. Historical Perspective of Microorganisms Involved in EBPR
3. Morphology, Physiology and Phylogeny of Tetrasphaera
3.1. Carbon Sources
3.2. Metabolic Models of EBPR
4. Occurrence of Tetrasphaera in EBPR Systems
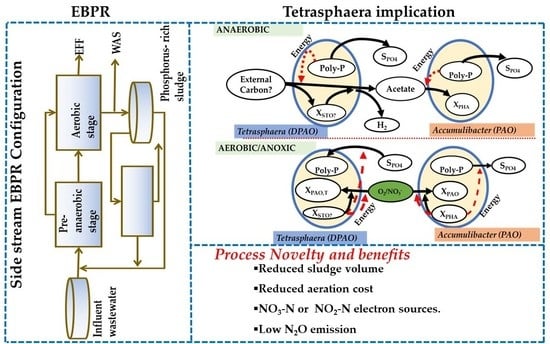
5. Implications of Tetrasphaera on EBPR Configuration and Operation
Configurations of the EBPR Systems
6. Factors Affecting the Occurrence and Activity of Tetrasphaera and Other DPAOs in EBPR Systems
6.1. Temperature
6.2. Influent Wastewater Characteristics
6.3. pH
6.4. Presence of DO and Nitrate
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metcalf and Eddy Inc. Wastewater Engineering Treatment and Reuse, 5th ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Barnard, J.L.; Dunlap, P.; Steichen, M. Rethinking the Mechanisms of Biological Phosphorus Removal. Water Environ. Res. 2017, 89, 2043–2054. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Saunders, A.M.; Hansen, A.A.; Larsen, P.; Nielsen, J.L. Microbial communities involved in enhanced biological phosphorus removal from wastewater—A model system in environmental biotechnology. Curr. Opin. Biotechnol. 2012, 23, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.H.; McIlroy, S.J.; Albertsen, M.; Nierychlo, M. Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr. Opin. Biotechnol. 2019, 57, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rincón, F.; Lopez-Vazquez, C.; Welles, L.; van Loosdrecht, M.; Brdjanovic, D. Cooperation between Candidatus Competibacter and Candidatus Accumulibacter clade I, in denitrification and phosphate removal processes. Water Res. 2017, 120, 156–164. [Google Scholar] [CrossRef]
- Marques, R.; Ribera-Guardia, A.; Santos, J.; Carvalho, G.; Reis, M.A.; Pijuan, M.; Oehmen, A. Denitrifying capabilities of Tetrasphaera and their contribution towards nitrous oxide production in enhanced biological phosphorus removal processes. Water Res. 2018, 137, 262–272. [Google Scholar] [CrossRef]
- Fernando, E.Y.; McIlroy, S.J.; Nierychlo, M.; Herbst, F.-A.; Petriglieri, F.; Schmid, M.C.; Wagner, M.; Nielsen, J.L.; Nielsen, P.H. Resolving the individual contribution of key microbial populations to enhanced biological phosphorus removal with Raman–FISH. ISME J. 2019, 13, 1933–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Kinyua, M.N. Identification and classification of the Tetrasphaera genus in enhanced biological phosphorus removal process: A review. Rev. Environ. Sci. Technol. 2020, 19, 699–715. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadi, P.; Eldyasti, A. Understanding microbial shift of Enhanced Biological Phosphorus Removal process (EBPR) under different Dissolved Oxygen (DO) concentrations and Hydraulic Retention Time (HRTs). Biochem. Eng. J. 2021, 166, 107833. [Google Scholar] [CrossRef]
- Kristiansen, R.; Nguyen, H.T.T.; Saunders, A.M.; Nielsen, J.L.; Wimmer, R.; Le, V.Q.; McIlroy, S.J.; Petrovski, S.; Seviour, R.J.; Calteau, A.; et al. A metabolic model for members of the genus Tetrasphaera involved in enhanced biological phosphorus removal. ISME J. 2013, 7, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Kristiansen, R.; Vestergaard, M.; Wimmer, R.; Nielsen, P.H. Intracellular Accumulation of Glycine in Polyphosphate-Accumulating Organisms in Activated Sludge, a Novel Storage Mechanism under Dynamic Anaerobic-Aerobic Conditions. Appl. Environ. Microbiol. 2015, 81, 4809–4818. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Guanglei, Q.; Zuniga-Montanez, R.; Williams, R.B.; Wuertz, S. Recent advances in understanding the ecophysiology of enhanced biological phosphorus removal. Curr. Opin. Biotechnol. 2021, 67, 166–174. [Google Scholar] [CrossRef]
- Liu, R.; Hao, X.; Chen, Q.; Li, J. Research advances of Tetrasphaera in enhanced biological phosphorus removal: A review. Water Res. 2019, 166, 115003. [Google Scholar] [CrossRef] [PubMed]
- Welles, L.; Tian, W.; Saad, S.; Abbas, B.; Lopez-Vazquez, C.; Hooijmans, C.; van Loosdrecht, M.; Brdjanovic, D. Accumulibacter clades Type I and II performing kinetically different glycogen-accumulating organisms metabolisms for anaerobic substrate uptake. Water Res. 2015, 83, 354–366. [Google Scholar] [CrossRef]
- Wisniewski, K.; Kowalski, M.; Makinia, J. Modeling nitrous oxide production by a denitrifying-enhanced biologically phosphorus removing (EBPR) activated sludge in the presence of different carbon sources and electron acceptors. Water Res. 2018, 142, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V.; Shapiro, J. Metabolic Uptake of Phosphorus by Wastewater Organisms. Water Pollut. Control. Fed. 1965, 37, 800–821. [Google Scholar]
- Srinath, E.G.; Sastry, C.A.; Pillai, S.C. Rapid removal of phosphorus from sewage by activated sludge. Experientia 1959, 15, 339–340. [Google Scholar] [CrossRef]
- Barnard, J.L. Cut P and N without chemicals. Water Wastes Eng. Part 1 1974, 11, 33–36. [Google Scholar]
- Barnard, J.L. Cut P and N without chemicals. Water Wastes Eng. Part 2 1974, 11, 41–43. [Google Scholar]
- Barnard, J.L. Nutrient removal in biological systems. Water Pollut. Control 1975, 74, 143–154. [Google Scholar]
- Levin, G.V.; Topol, G.J.; Tarnay, A.G. Operation of full-scale biological phosphorus removal plant. Water Pollut. Control Fed. 1975, 47, 577–590. [Google Scholar]
- Fuhs, G.W.; Chen, M. Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater. Microb. Ecol. 1975, 2, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Loy, A.; Nogueira, R.; Purkhold, U.; Valeeva, A.V.; Daims, H. Microbial community composition and function in wastewater treatment plants. Antonie Leeuwenhoek 2002, 81, 665–680. [Google Scholar] [CrossRef]
- Bond, P.L.; Hugenholtz, P.; Keller, J.; Blackall, L.L. Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors. Appl. Environ. Microbiol. 1995, 61, 1910–1916. [Google Scholar] [CrossRef] [Green Version]
- Bond, P.L.; Keller, J.; Blackall, L.L. Characterisation of enhanced biological phosphorus removal activated sludges with dissimilar phosphorus removal performances. Water Sci. Technol. 1998, 37, 567–571. [Google Scholar] [CrossRef]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.J.; Saunders, A.; Albertsen, M.; Nierychlo, M.; McIlroy, B.; Hansen, A.A.; Karst, S.M.; Nielsen, J.L.; Nielsen, P.H. MiDAS: The field guide to the microbes of activated sludge. Database 2015, 2015, bav062. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; van Loosdrecht, M. Activated Sludge Models, ASM1, ASM2, ASM2d and ASM3. Scientific and Technical Report (Volume 5); IWA Publishing: London, UK, 2000. [Google Scholar] [CrossRef]
- Jenkins, D.; Wanner, J. Activated Sludge—100 Years and Counting; IWA Publishing: Glasgow, UK, 2014. [Google Scholar] [CrossRef]
- Bertanza, G.; Menoni, L.; Capoferri, G.U.; Pedrazzani, R. Promoting biological phosphorus removal in a full scale pre-denitrification wastewater treatment plant. J. Environ. Manag. 2020, 254, 109803. [Google Scholar] [CrossRef]
- Hanada, S.; Liu, W.-T.; Shintani, T.; Kamagata, Y.; Nakamura, K. Tetrasphaera elongata sp. nov., a polyphosphate-accumulating bacterium isolated from activated sludge. Int. J. Syst. Evol. Microbiol. 2002, 52, 883–887. [Google Scholar] [CrossRef]
- Maszenan, A.; Seviour, R.; Patel, B.; Schumann, P.; Burghardt, J.; Tokiwa, Y.; Stratton, H. Three isolates of novel polyphosphate-accumulating gram-positive cocci, obtained from activated sludge, belong to a new genus, Tetrasphaera gen. nov., and description of two new species, Tetrasphaera japonica sp. nov. and Tetrasphaera australiensis sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Stokholm-Bjerregaard, M.; McIlroy, S.J.; Nierychlo, M.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. A Critical Assessment of the Microorganisms Proposed to be Important to Enhanced Biological Phosphorus Removal in Full-Scale Wastewater Treatment Systems. Front. Microbiol. 2017, 8, 718. [Google Scholar] [CrossRef] [Green Version]
- Seviour, R.J.; Mino, T.; Onuki, M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 2003, 27, 99–127. [Google Scholar] [CrossRef] [Green Version]
- Seviour, R.J.; McIlroy, S.J. The microbiology of phosphorus removal in activated sludge processes-the current state of play. J. Microbiol. 2008, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Petriglieri, F.; Singleton, C.; Peces, M.; Petersen, J.F.; Nierychlo, M.; Nielsen, P.H. “Candidatus Dechloromonas phosphatis” and “Candidatus Dechloromonas phosphovora”, two novel polyphosphate accumulating organisms abundant in wastewater treatment systems. BioRxiv 2020. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Le-Quy, V.; Hansen, A.A.; Nielsen, J.L.; Nielsen, P.H. High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems. FEMS Microbiol. Ecol. 2011, 76, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.; Santos, J.; Nguyen, H.; Carvalho, G.; Noronha, J.; Nielsen, P.H.; Reis, M.A.; Oehmen, A. Metabolism and ecological niche of Tetrasphaera and Ca. Accumulibacter in enhanced biological phosphorus removal. Water Res. 2017, 122, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Singleton, C.M.; Petriglieri, F.; Wasmund, K.; Nierychlo, M.; Kondrotaite, Z.; Petersen, J.F.; Peces, M.; Dueholm, M.S.; Wagner, M.; Nielsen, P.H. The novel genus, ‘Candidatus Phosphoribacter’, previously identified as Tetrasphaera, is the dominant polyphosphate accumulating lineage in EBPR wastewater treatment plants worldwide. ISME J. 2022, 16, 1605–1616. [Google Scholar] [CrossRef]
- Nouioui, I.; Carro, L.; García-López, M.; Meier-Kolthoff, J.P.; Woyke, T.; Kyrpides, N.C.; Pukall, R.; Klenk, H.-P.; Goodfellow, M.; Göker, M. Genome-Based Taxonomic Classification of the Phylum Actinobacteria. Front. Microbiol. 2018, 9, 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Close, K.; Marques, R.; Carvalho, V.C.; Freitas, E.B.; Reis, M.A.; Carvalho, G.; Oehmen, A. The storage compounds associated with Tetrasphaera PAO metabolism and the relationship between diversity and P removal. Water Res. 2021, 204, 117621. [Google Scholar] [CrossRef]
- Kong, Y.; Nielsen, J.L.; Nielsen, P.H. Identity and Ecophysiology of Uncultured Actinobacterial Polyphosphate-Accumulating Organisms in Full-Scale Enhanced Biological Phosphorus Removal Plants. Appl. Environ. Microbiol. 2005, 71, 4076–4085. [Google Scholar] [CrossRef] [Green Version]
- Mino, T.; Arun, V.; Tsuzuki, Y.; Matsuo, T. Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process. In Biological Phosphate Removal from Wastewaters, Proceedings of an IAWPRC Specialized Conference held in Rome, Italy, 28–30 September 1987; Ramadori, R., Ed.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 27–38. [Google Scholar] [CrossRef]
- Smolders, G.J.F.; van der Meij, J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Model of the anaerobic metabolism of the biological phosphorus removal process: Stoichiometry and pH influence. Biotechnol. Bioeng. 1994, 43, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Filipe, C.D.M.; Daigger, G.T.; Grady, C.P.L. A metabolic model for acetate uptake under anaerobic conditions by glycogen accumulating organisms: Stoichiometry, kinetics, and the effect of pH. Biotechnol. Bioeng. 2001, 76, 17–31. [Google Scholar] [CrossRef]
- Vlekke, G.; Comeau, Y.; Oldham, W. Biological phosphate removal from wastewater with oxygen or nitrate in sequencing batch reactors. Environ. Technol. Lett. 1988, 9, 791–796. [Google Scholar] [CrossRef]
- Mąkinia, J.; Zaborowska, E. Mathematical Modelling and Computer Simulation of Activated Sludge Systems; International Water Association Publishing: London, UK, 2020. [Google Scholar] [CrossRef]
- Izadi, P.; Eldyasti, A. A review of biochemical diversity and metabolic modeling of EBPR process under specific environmental conditions and carbon source availability. J. Environ. Manag. 2021, 288, 112362. [Google Scholar] [CrossRef]
- Oehmen, A.; Carvalho, G.; Lopez-Vazquez, C.; van Loosdrecht, M.; Reis, M. Incorporating microbial ecology into the metabolic modelling of polyphosphate accumulating organisms and glycogen accumulating organisms. Water Res. 2010, 44, 4992–5004. [Google Scholar] [CrossRef]
- Lopez-Vazquez, C.M.; Oehmen, A.; Hooijmans, C.M.; Brdjanovic, D.; Gijzen, H.J.; Yuan, Z.; van Loosdrecht, M.C. Modeling the PAO–GAO competition: Effects of carbon source, pH and temperature. Water Res. 2009, 43, 450–462. [Google Scholar] [CrossRef]
- Herbst, F.-A.; Dueholm, M.S.; Wimmer, R.; Nielsen, P.H. The Proteome of Tetrasphaera elongata is adapted to Changing Conditions in Wastewater Treatment Plants. Proteomes 2019, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Meinhold, J.; Filipe, C.D.; Daigger, G.T.; Isaacs, S.H. Characterization of the denitrifying fraction of phosphate accumulating organisms in biological phosphate removal. Water Sci. Technol. 1999, 39, 31–42. [Google Scholar] [CrossRef]
- Mino, T.; van Loosdrecht, M.; Heijnen, J. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 1998, 32, 3193–3207. [Google Scholar] [CrossRef]
- Meng, Q.; Zeng, W.; Wang, B.; Fan, Z.; Peng, Y. New insights in the competition of polyphosphate-accumulating organisms and glycogen-accumulating organisms under glycogen accumulating metabolism with trace Poly-P using flow cytometry. Chem. Eng. J. 2020, 385, 123915. [Google Scholar] [CrossRef]
- Schuler, A.J.; Jenkins, D. Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, Part I: Experimental results and comparison with metabolic models. Water Environ. Res. 2003, 75, 485–498. [Google Scholar] [CrossRef]
- Winkler, M.-K.; Bassin, J.; Kleerebezem, R.; de Bruin, L.; Brand, T.V.D.; van Loosdrecht, M. Selective sludge removal in a segregated aerobic granular biomass system as a strategy to control PAO–GAO competition at high temperatures. Water Res. 2011, 45, 3291–3299. [Google Scholar] [CrossRef]
- Wong, M.-T.; Tan, F.M.; Ng, W.J.; Liu, W.-T. Identification and occurrence of tetrad-forming Alphaproteobacteria in anaerobic–aerobic activated sludge processes. Microbiology 2004, 150, 3741–3748. [Google Scholar] [CrossRef] [Green Version]
- Carvalheira, M.; Oehmen, A.; Carvalho, G.; Reis, M.A. The effect of substrate competition on the metabolism of polyphosphate accumulating organisms (PAOs). Water Res. 2014, 64, 149–159. [Google Scholar] [CrossRef]
- Onnis-Hayden, A.; Majed, N.; Li, Y.; Rahman, S.M.; Drury, D.; Risso, L.; Gu, A.Z. Impact of solid residence time (SRT) on functionally relevant microbial populations and performance in full-scale enhanced biological phosphorus removal (EBPR) systems. Water Environ. Res. 2020, 92, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Majed, N.; Gu, A.Z. Phenotypic dynamics in polyphosphate and glycogen accumulating organisms in response to varying influent C/P ratios in EBPR systems. Sci. Total Environ. 2020, 743, 140603. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Zhou, Y. Enhanced biological phosphorus removal with different carbon sources. Appl. Microbiol. Biotechnol. 2016, 100, 4735–4745. [Google Scholar] [CrossRef]
- Oyserman, B.; Noguera, D.R.; del Rio, T.G.; Tringe, S.G.; McMahon, K.D. Metatranscriptomic insights on gene expression and regulatory controls in Candidatus Accumulibacter phosphatis. ISME J. 2016, 10, 810–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.; Schuler, A.J. Low Acetate Concentrations Favor Polyphosphate-Accumulating Organisms over Glycogen-Accumulating Organisms in Enhanced Biological Phosphorus Removal from Wastewater. Environ. Sci. Technol. 2013, 47, 3816–3824. [Google Scholar] [CrossRef]
- Izadi, P.; Eldyasti, A. Design, operation and technology configurations for enhanced biological phosphorus removal (EBPR) process: A review. Rev. Environ. Sci. Technol. 2020, 19, 561–593. [Google Scholar] [CrossRef]
- Barnard, J.L. A review of biological phosphorus removal in the activated sludge process. Water SA 1976, 2, 136–144. [Google Scholar]
- Alasino, N.; Mussati, M.C.; Scenna, N.; Aguirre, P. Combined nitrogen and phosphorus removal. Model-based process optimization. Comput. Aided Chem. Eng. 2008, 25, 163–168. [Google Scholar] [CrossRef]
- Guerrero, J.; Guisasola, A.; Baeza, J.A. A novel control strategy for efficient biological phosphorus removal with carbon-limited wastewaters. Water Sci. Technol. 2014, 70, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Flores-Alsina, X.; Guisasola, A.; Baeza, J.A.; Gernaey, K.V. Effect of nitrite, limited reactive settler and plant design configuration on the predicted performance of simultaneous C/N/P removal WWTPs. Bioresour. Technol. 2013, 136, 680–688. [Google Scholar] [CrossRef] [Green Version]
- Conidi, D.; Andalib, M.; Andres, C.; Bye, C.; Umble, A.; Dold, P. Modeling quaternary ammonium compound inhibition of biological nutrient removal activated sludge. Water Sci. Technol. 2018, 79, 41–50. [Google Scholar] [CrossRef]
- Onnis-Hayden, A.; Srinivasan, V.; Tooker, N.B.; Li, G.; Wang, D.; Barnard, J.L.; Bott, C.; Dombrowski, P.; Schauer, P.; Menniti, A.; et al. Survey of full-scale sidestream enhanced biological phosphorus removal (S2EBPR) systems and comparison with conventional EBPRs in North America: Process stability, kinetics, and microbial populations. Water Environ. Res. 2020, 92, 403–417. [Google Scholar] [CrossRef]
- Dold, P.; Conidi, D. Achieving Enhanced Biological P Removal: Have we forgotten how to design a bioP plant? In Proceedings of the 92nd Annual Water Environment Federation Technical Exhibition and Conference [CD-ROM], Chicago, IL, USA, 21–25 September 2019; pp. 1452–1466. [Google Scholar]
- Gu, A.Z.; Tooker, N.; Onnis-Hayden, A.; Wang, D.; Srinivasan, V.; Li, G.; Takács, I.; Vargas, E. Optimization and Design of a Side-Stream EBPR Process as a Sustainable Approach for Achieving Stable and Efficient Phosphorus Removal; Project No. U1R13/4869; WRF: Alexandria, VA, USA, 2019. [Google Scholar]
- Ferrera, I.; Sánchez, O. Insights into microbial diversity in wastewater treatment systems: How far have we come? Biotechnol. Adv. 2016, 34, 790–802. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Wu, D.; Hao, T.; Mackey, H.R.; Wei, L.; Chen, G. Denitrifying sulfur conversion-associated EBPR: The effect of pH on anaerobic metabolism and performance. Water Res. 2017, 123, 687–695. [Google Scholar] [CrossRef]
- Mulkerrins, D.; Dobson, A.; Colleran, E. Parameters affecting biological phosphate removal from wastewaters. Environ. Int. 2004, 30, 249–259. [Google Scholar] [CrossRef]
- Gebremariam, S.Y.; Beutel, M.W.; Christian, D.; Hess, T.F. Research Advances and Challenges in the Microbiology of Enhanced Biological Phosphorus Removal—A Critical Review. Water Environ. Res. 2011, 83, 195–219. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, M.; Wang, Y.; Liu, F.; Qin, M.; Zhang, Y.; Zhou, W. The condition optimization and mechanism of aerobic phosphorus removal by marine bacterium Shewanella sp. Chem. Eng. J. 2018, 345, 611–620. [Google Scholar] [CrossRef]
- Panswad, T.; Doungchai, A.; Anotai, J. Temperature effect on microbial community of enhanced biological phosphorus removal system. Water Res. 2003, 37, 409–415. [Google Scholar] [CrossRef]
- Weissbrodt, D.G.; Neu, T.R.; Kuhlicke, U.; Rappaz, Y.; Holliger, C. Assessment of bacterial and structural dynamics in aerobic granular biofilms. Front. Microbiol. 2013, 4, 175. [Google Scholar] [CrossRef] [Green Version]
- Whang, L.-M.; Park, J.K. Competition between Polyphosphate- and Glycogen-Accumulating Organisms in Enhanced-Biological-Phosphorus-Removal Systems: Effect of Temperature and Sludge Age. Water Environ. Res. 2006, 78, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.K.; Ong, Y.H.; Arumugam, K.; Nittami, T.; Yeoh, H.K.; Bessarab, I.; William, R.; Chua, A.S.M. Tropical-based EBPR process: The long-term stability, microbial community and its response towards temperature stress. Water Environ. Res. 2021, 93, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Zuniga-Montanez, R.; Law, Y.; Thi, S.S.; Nguyen, T.Q.N.; Eganathan, K.; Liu, X.; Nielsen, P.H.; Williams, R.B.; Wuertz, S. Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources. Water Res. 2019, 149, 496–510. [Google Scholar] [CrossRef]
- Wang, L.; Shen, N.; Oehmen, A.; Zhou, Y. The impact of temperature on the metabolism of volatile fatty acids by polyphosphate accumulating organisms (PAOs). Environ. Res. 2020, 188, 109729. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, A.T.; Nguyen, H.T.; Nielsen, J.L.; Nielsen, P.H. Population dynamics of bacteria involved in enhanced biological phosphorus removal in Danish wastewater treatment plants. Water Res. 2013, 47, 1529–1544. [Google Scholar] [CrossRef]
- Liau, K.F.; Shoji, T.; Ong, Y.H.; Chua, A.S.M.; Yeoh, H.K.; Ho, P.Y. Kinetic and stoichiometric characterization for efficient enhanced biological phosphorus removal (EBPR) process at high temperatures. Bioprocess Biosyst. Eng. 2015, 38, 729–737. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, H.; Liu, J.; Luo, J.; Qian, G.; Wang, A. pH dependent phosphorus release from waste activated sludge: Contributions of phosphorus speciation. Chem. Eng. J. 2015, 267, 260–265. [Google Scholar] [CrossRef]
- Wang, D.; Tooker, N.B.; Srinivasan, V.; Li, G.; Fernandez, L.A.; Schauer, P.; Menniti, A.; Maher, C.; Bott, C.B.; Dombrowski, P.; et al. Side-stream enhanced biological phosphorus removal (S2EBPR) process improves system performance—A full-scale comparative study. Water Res. 2019, 167, 115109. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.J.; Munz, G.; Yuan, Q. Influence of pH control on material characteristics, bacterial community composition and BNR performance of mature aerobic granules. Process Saf. Environ. Prot. 2019, 124, 158–166. [Google Scholar] [CrossRef]
- Filipe, C.D.; Daigger, G.T.; Grady, C.L. pH as a key factor in the competition between glycogen-accumulating organisms and phosphorus-accumulating organisms. Water Environ. Res. 2001, 73, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Mabbett, A.N.; McEwan, A.G.; Blackall, L.L. Proton motive force generation from stored polymers for the uptake of acetate under anaerobic conditions. FEMS Microbiol. Lett. 2007, 274, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belka, D. The Effect of pH on Organic Carbon Uptake and Biological Phosphorus Removal by Tetrasphaera Polyphosphate Accumulating Organisms. 2021. Available online: https://digitalrepository.unm.edu/ce_etds/259 (accessed on 8 October 2022).
- Zuthi, M.; Guo, W.; Ngo, H.; Nghiem, L.; Hai, F. Enhanced biological phosphorus removal and its modeling for the activated sludge and membrane bioreactor processes. Bioresour. Technol. 2013, 139, 363–374. [Google Scholar] [CrossRef]
- Chen, H.-B.; Wang, D.-B.; Li, X.-M.; Yang, Q.; Luo, K.; Zeng, G.-M. Temperature influence on biological phosphorus removal induced by aerobic/extended-idle regime. Environ. Sci. Pollut. Res. 2014, 21, 6034–6043. [Google Scholar] [CrossRef]





| Bacterial Functional Group | Electron Acceptors |
|---|---|
| APAO | DO |
| DPAO I | NO3−→NO2−→N2O→N2 |
| DPAO II * | NO2−→N2O→N2 |
| GAO | NO3−→NO2−→N2O→N2 |
| T. australiensis T. japonica T. elongata | NO3−→NO2−→N2O→N2 |
| T. jenkinsii, T. vanveenii, T. veronensis | DO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otieno, J.; Kowal, P.; Mąkinia, J. The Occurrence and Role of Tetrasphaera in Enhanced Biological Phosphorus Removal Systems. Water 2022, 14, 3428. https://doi.org/10.3390/w14213428
Otieno J, Kowal P, Mąkinia J. The Occurrence and Role of Tetrasphaera in Enhanced Biological Phosphorus Removal Systems. Water. 2022; 14(21):3428. https://doi.org/10.3390/w14213428
Chicago/Turabian StyleOtieno, Jeremiah, Przemysław Kowal, and Jacek Mąkinia. 2022. "The Occurrence and Role of Tetrasphaera in Enhanced Biological Phosphorus Removal Systems" Water 14, no. 21: 3428. https://doi.org/10.3390/w14213428