Isotopic Evidence for Anaerobic Oxidation of Methane in the Freshwater Sediments of Reservoirs: The Impact of Selected Environmental Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.2.1. Sediment Sampling and Preparation
2.2.2. Sediment Analysis
2.2.3. Pore-Water Analysis
2.2.4. 13CH4 Incubation Experiment
2.2.5. Calculations
3. Results
3.1. Sediment Characteristics
3.2. Pore-Water Characteristics
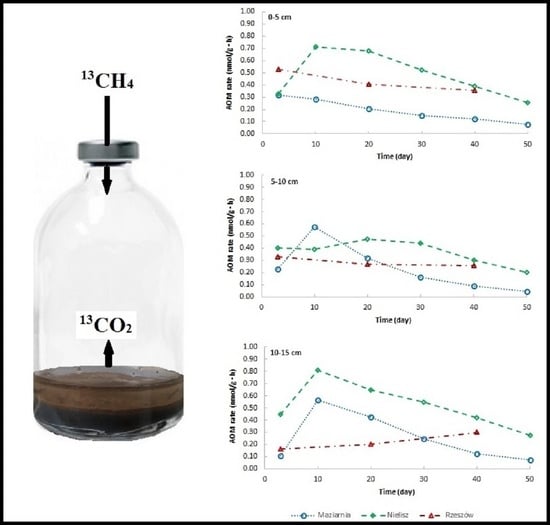
3.3. Rate of AOM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. [Google Scholar]
- Downing, J.A.; Prairie, Y.T.; Cole, J.J.; Duarte, C.M.; Tranvik, L.J.; Striegl, R.G.; McDowell, W.H.; Kortelainen, P.; Caraco, N.F.; Melack, J.M.; et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr. 2006, 51, 2388–2397. [Google Scholar] [CrossRef] [Green Version]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.M.; Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science 2011, 331, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deemer, B.R.; Harrison, J.A.; Li, S.; Beaulieu, J.J.; DelSontro, T.; Barros, N.; Bezerra-Neto, J.F.; Powers, S.M.; Dos Santos, M.A.; Vonk, J.A. Greenhouse gas emissions from reservoir water surfaces: A new global synthesis. BioScience 2016, 66, 949–964. [Google Scholar] [CrossRef] [Green Version]
- DelSontro, T.; Beaulieu, J.J.; Downing, J.A. Greenhouse gas emissions from lakes and impoundments: Upscaling in the face of global change. Limnol. Oceanogr. Lett. 2018, 3, 64–75. [Google Scholar] [CrossRef]
- Gruca-Rokosz, R. Quantitative fluxes of the greenhouse gases CH4 and CO2 from the surfaces of selected Polish reservoirs. Atmosphere 2020, 11, 286. [Google Scholar] [CrossRef] [Green Version]
- Soued, C.; Prairie, Y.T. Changing sources and processes sustaining surface CO2 and CH4 fluxes along a tropical river to reservoir system. Biogeosciences 2021, 18, 1333–1350. [Google Scholar] [CrossRef]
- DelSontro, T.; Kunz, M.J.; Kempter, T.; Wuest, A.; Wehrli, B.; Senn, D.B. Spatial heterogeneity of methane ebullition in a large tropical reservoir. Environ. Sci. Technol. 2011, 45, 9866–9873. [Google Scholar] [CrossRef] [PubMed]
- Sobek, S.; DelSontro, T.; Wongfun, N.; Wehrli, B. Extreme organic carbon burial fuels intense methane bubbling in a temperate reservoir. Geophys. Res. Lett. 2012, 39, L01401. [Google Scholar] [CrossRef] [Green Version]
- Maeck, A.; DelSontro, T.; McGinnis, D.F.; Fischer, H.; Flury, S.; Schmidt, M.; Fietzek, P.; Lorke, A. Sediment trapping by dams creates methane emission hot spots. Environ. Sci. Technol. 2013, 47, 8130–8137. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; Smolenski, R.L.; Nietch, C.T.; Townsend-Small, A.; Elovitz, M.S. High methane emissions from a midlatitude reservoir draining an agricultural watershed. Environ. Sci. Technol. 2014, 48, 11100–11108. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; McManus, M.G.; Nietch, C.T. Estimates of reservoir methane emissions based on a spatially balanced probabilistic-survey. Limnol. Oceanogr. 2016, 61, S27–S40. [Google Scholar] [CrossRef] [Green Version]
- Berberich, M.E.; Beaulieu, J.J.; Hamilton, T.L.; Waldo, S.; Buffam, I. Spatial variability of sediment methane production and methanogen communities within a eutrophic reservoir: Importance of organic matter source and quantity. Limnol. Oceanogr. 2019, 9999, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Xu, Q.; Li, H.; Cheng, C.; He, Q. Lack of methane hotspot in the upstream dam: Case study in a tributary of the Three Gorges Reservoir, China. Sci. Total. Environ. 2021, 754, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Gruca-Rokosz, R.; Cieśla, M. Sediment methane production within eutrophic reservoirs: The importance of sedimenting organic matter. Sci. Total. Environ. 2021, 799, 149219. [Google Scholar] [CrossRef] [PubMed]
- Raghoebarsing, A.A.; Pol, A.; van de Pas-Schoonen, K.T.; Smolders, A.J.; Ettwig, K.F.; Rijpstra, W.I.; Schouten, S.; Damsté, J.S.; Op den Camp, H.J.; Jetten, M.S.; et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 2006, 440, 918–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettwig, K.F.; Shima, S.; de van Pas-Schoonen, K.T.; Kahnt, J.; Medema, M.H.; Op den Camp, H.J.; Jetten, M.S.; Strous, M. Denitrifying bacteria anaerobically oxidize methane in the absence of archaea. Environ. Microbiol. 2008, 10, 3164–3173. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.J.; House, C.H.; Orphan, V.J. Manganese-and iron-dependent marine methane oxidation. Science 2009, 3255937, 184–187. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, E.I.; Avendaño, K.A.; Balagurusamy, N.; Arriaga, S.; Nieto-Delgado, C.; Thalasso, F.; Cervantes, F.J. Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments. Sci. Total. Environ. 2019, 650, 2674–2684. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Prieto-Davó, A.; López Lozano, N.E.; Hernández-Eligio, A.; Vega-Alvarado, L.; Juárez, K.; García-González, A.S.; López, M.G.; Cervantes, F.J. Anaerobic methane oxidation driven by microbial reduction of natural organic matter in a tropical wetland. Appl. Environ. Microbiol. 2017, 83, e00645-17. [Google Scholar] [CrossRef] [Green Version]
- Ettwig, K.F.; Butler, M.K.; Le Paslier, D.; Pelletier, E.; Mangenot, S.; Kuypers, M.M.; Schreiber, F.; Dutilh, B.E.; Zedelius, J.; de Beer, D.; et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 2010, 4647288, 543–548. [Google Scholar] [CrossRef]
- Timmers, P.H.; Welte, C.U.; Koehorst, J.J.; Plugge, C.M.; Jetten, M.S.; Stams, A.J. Reverse methanogenesis and respiration in methanotrophic archaea. Archaea 2017, 2017, 1654237. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Leu, A.O.; Xie, G.; Guo, J.; Feng, Y.; Zhao, J.; Tyson, G.W.; Yuan, Z.; Hu, S. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J. 2018, 12, 1929–1939. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wu, J.; Lu, Y.; Fu, L.; Zhang, F.; Lau, T.; Zeng, R.J. Humic substances as electron acceptors for anaerobic oxidation of methane driven by ANME-2d. Water Res. 2019, 164, 114935. [Google Scholar] [CrossRef] [PubMed]
- Brocks, J.J.; Summons, R.E. Sedimentary Hydrocarbons, Biomarkers for Early Life. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 8, pp. 63–115. [Google Scholar]
- Blumenberg, M.; Seifert, R.; Reitner, J.; Pape, T.; Michaelis, W. Membrane lipid patterns typify distinct anaerobic methanotrophic consortia. Proc. Natl. Acad. Sci. USA 2004, 101, 11111–11116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadnitskaia, A.; Muyzer, G.; Abbas, B.; Coolen, M.J.L.; Hopmans, E.C.; Baas, M.; Van Weering, T.C.E.; Ivanov, M.K.; Poludetkina, E.; Damste, J.S.S. Biomarker and 16S rDNA evidence for anaerobic oxidation of methane and related carbonate precipitation in deep-sea mud volcanoes of the Sorokin Trough, Black Sea. Mar. Geol. 2015, 217, 67–96. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, S. Acyclic hydrocarbons and ketones in cold-seep carbonates from central Hokkaido, northern Japan. Geochem. J. 2010, 42, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V. Anaerobic Oxidation of Methane in Northern Peatland. Master Thesis, Department of Geography, University of Toronto, Toronto, ON, Canada, 2011; pp. 1–103. [Google Scholar]
- Hu, B.L.; Shen, L.D.; Lian, X.; Zhu, Q.; Liu, S.; Huang, Q.; He, Z.F.; Geng, S.; Cheng, D.Q.; Lou, L.P.; et al. Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc. Natl. Acad. Sci. USA 2014, 111, 4495–4500. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, Z.; He, C.; Zhang, X.; Sheng, L.; Ren, X. Using 13C isotopes to explore denitrification-dependent anaerobic methane oxidation in a paddy-peatland. Sci. Rep. 2017, 7, 40848. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Ouyang, L.; Zhu, Y.; Trimmer, M. Active pathways of anaerobic methane oxidation across contrasting riverbeds. ISME J. 2019, 13, 752–766. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Dippold, M.A.; Ge, T.; Wu, J.; Thiel, V.; Kuzyakov, Y.; Dorodnikow, M. Anaerobic oxidation of methane in paddy soil: Role of electron acceptors and fertilization in mitigating CH4 fluxes. Soil Biol. Biochem. 2020, 141, 107685. [Google Scholar] [CrossRef]
- Gruca-Rokosz, R. Dynamika Węglowych Gazów Cieplarnianych w Zbiornikach Zaporowych—Mechanizmy Produkcji, Emisja do Atmosfery; Oficyna Wydawnicza Politechniki Rzeszowskiej: Rzeszów, Poland, 2015; pp. 1–132. [Google Scholar]
- Gruca-Rokosz, R.; Tomaszek, J. Methane and carbon dioxide in the sediment of a eutrophic reservoir: Production pathways and diffusion fluxes at the sediment-water interface. Water Air Soil Pollut. 2015, 226, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruca-Rokosz, R.; Koszelnik, P. Production pathways for CH4 and CO2 in sediments of two freshwater ecosystems in south-eastern Poland. PLoS ONE 2018, 13, e0199755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottom sediment sampler. Patent no.239528, 2021.
- Reeburgh, W.S. An improved interstitial water sampler. Limnol. Oceanogr. 1967, 12, 163–165. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, C.F.; Keefe, C.W.; Bashe, J. Determination of Carbon and Nitrogen in Sediments and Particulates/Coastal Waters Using Elemental Analysis; Method 440.0; NER Laboratory, USEPA: Cincinnati, OH, USA, 1997. [Google Scholar]
- Griffith, S.M.; Schnitzer, M. Analytical characteristics of humic and fulvic acids extracted from tropical volcanic soils. Soil Sei. Soc. Am. Proc. 1975, 39, 861–867. [Google Scholar] [CrossRef]
- Hinrichs, K.-U.; Pancost, R.D.; Summons, R.E.; Sprott, G.D.; Sylva, S.P.; Sinninghe Damste, J.S.; Hayes, J.M. Mass spectra ofsn-2-hydroxyarchaeol, a polar lipid biomarker for anaerobic methanotrophy. Geochem. Geophys. Geosyst. 2000, 1, 2000GC000042. [Google Scholar] [CrossRef]
- Elvert, M.; Hopmans, E.C.; Treude, T.; Boetius, A.; Suess, E. Spatial variations of methanotrophic consortia at cold methane seeps: Implications from a high-resolution molecular and isotopic approach. Geobiology 2005, 3, 195–209. [Google Scholar] [CrossRef]
- Niemann, H.; Elvert, M.; Hovland, M.; Orcutt, B.; Judd, A.; Suck, I.; Gutt, J.; Joye, S.; Damm, E.; Finster, K.; et al. Methane emission and consumption at a North Sea gas seep (Tommeliten area). Biogeosciences 2005, 2, 335–351. [Google Scholar] [CrossRef] [Green Version]
- Niemann, H.; Elvert, M. Diagnostic lipid biomarker and stable carbon isotope signatures of microbial communities mediating the anaerobic oxidation of methane with sulphate. Org. Geochem. 2008, 39, 1668–1677. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Aoki, M.; Ehara, M.; Saito, Y.; Yoshioka, H.; Miyazaki, M.; Saito, Y.; Miyashita, A.; Kawakami, S.; Yamaguchi, T.; Ohashi, A.; et al. A long-term cultivation of an anaerobic methane-oxidizing microbial community from deep-sea methane-seep sediment using a continuous-flow bioreactor. PLoS ONE 2014, 9, e105356. [Google Scholar] [CrossRef]
- Meulepas, R.J.W.; Jagersma, C.G.; Gieteling, J.; Buisman, C.J.N.; Stams, A.J.M.; Lens, P.N.L. Enrichment of anaerobic methanotrophs in a sulfate-reducing membrane bioreactors. Biotechnol. Bioeng. 2009, 104, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Jagersma, G.C.; Meulepas, R.J.W.; Jong, I.H.; Gieteling, J.; Klimiuk, A.; Schouten, S.; Damsté, J.S.S.; Lens, P.N.L.; Stams, A.J.M. Microbial diversity and community structure of a highly active anaerobic methane-oxidizing sulfate-reducing enrichment. Environ. Microbiol. 2009, 12, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Smemo, K.A.; Yavitt, J.; Fowle, D.A.; Branfireun, B.A.; Basiliko, N. Stable isotopes reveal widespread anaerobic methane oxidation across latitude and peatland type. Environ. Sci. Technol. 2013, 47, 8273–8279. [Google Scholar] [CrossRef] [PubMed]
- Szal, D.; Gruca-Rokosz, R. Anaerobic oxidation of methane in freshwater sediments of Rzeszów Reservoir. Water 2020, 12, 398. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xue, S.; Xi, J. Anaerobic oxidation of methane coupled to sulfate reduction: Consortium characteristics and application in co-removal of H2S and methane. J. Environ. Sci. 2019, 76, 238–248. [Google Scholar] [CrossRef]
- Cassarini, C. Anaerobic Oxidation of Methane Coupled to Reduction of Different Sulfur Compounds as Electron Acceptors in Bioreactors; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Schubert, C.J.; Vazquez, F.; Lösekann-Behrens, T.; Knittel, K.; Tonolla, M.; Boetius, A. Evidence for anaerobic oxidation of methane in sediments of a freshwater system (Lago di Cadagno). FEMS Microbiol. Ecol. 2011, 76, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Winkel, M.; Sepulveda-Jauregui, A.; Martinez-Cruz, K.; Heslop, J.K.; Rijkers, R.; Horn, F. First evidence for cold-adapted anaerobic oxidation of methane in deep sediments of thermokarst lakes. Environ. Res. Commun. 2019, 1, 021002. [Google Scholar] [CrossRef]
- Smemo, K.; Yavitt, J.B. Evidence for anaerobic CH4 oxidation in freshwater peatlands. Geomicrobiology 2007, 24, 583–597. [Google Scholar] [CrossRef]
- Blazewicz, S.; Petersen, D.; Waldrop, M.; Firestone, M. Anaerobic oxidation of methane in tropical and boreal soils: Ecological significance in terrestrial methane cycling. J. Geophys. Res. 2012, 117, G02033. [Google Scholar] [CrossRef]
- Nordi, K.; Thamdrup, B.; Schubert, C. Anaerobic oxidation of methane in an iron-rich Danish freshwater lake sediment. Limnol. Oceanogr. 2013, 58, 546–554. [Google Scholar] [CrossRef]
- Saarela, T.; Rissanen, A.; Ojala, A.; Pumpanen, J.; Aalto, S.; Tiirola, M.; Vesala, T.; Jantti, H. CH4 oxidation in a boreal lake during the development of hypolimnetic hypoxia. Aquat. Sci. 2020, 82, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smemo, K.A. Methane Cycling in Northern Peatland Ecosystems. A Potential Role for Anaerobic Methane Oxidation. PhD. Thesis, Cornell University, Ithaca, NY, USA, 2003; pp. 1–138. [Google Scholar]
- Lovley, D.R.; Klug, M.J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl. Environ. Microbiol. 1983, 45, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, T.J.; Grisewood, M.J.; Nazem-Bokaee, H.; Gopalakrishnan, S.; Ferry, J.G.; Wood, T.K.; Maranas, C.D. Methane oxidation by anaerobic archaea for conversion to liquid fuels. J. Ind. Microbiol. Biotechnol. 2015, 42, 391–401. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Q.; Feng, Y.; Luo, H.; Pan, X.; Michael, G. Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane. Sci. Total. Environ. 2018, 610, 759–768. [Google Scholar] [CrossRef]
- Blodau, C.; Deppe, M. Humic acid addition lowers methane release in peats of the Mer Bleue bog, Canada. Soil Biol. Biochem. 2012, 52, 96–98. [Google Scholar] [CrossRef]
- Gruca-Rokosz, R.; Szal, D.; Bartoszek, L.; Pękala, A. Isotopic evidence for vertical diversification of methane production pathways in freshwater sediments of Nielisz reservoir (Poland). Catena 2020, 195, 104803. [Google Scholar] [CrossRef]
- Pancost, R.D.; McClymont, E.L.; Bingham, E.M.; Roberts, Z.; Charman, D.; Hornibrook, E.R.C.; Blundell, A.; Chambers, F.M.; Lim, K.L.H.; Evershed, R.P. Archaeol as a methanogen biomarker in ombrotrophic bogs. Org. Geochem. 2011, 42, 1279–1287. [Google Scholar] [CrossRef]
- Pancost, R.D.; Zhang, C.L.; Tavacoli, J.; Talbot, H.M.; Farrimond, P.; Schouten, S.; Damste, J.S.S.; Sassen, R. Lipid biomarkers perserved in hydrate-associated authigenic carbonate rocks of the Gulf of Mexico. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 227, 48–66. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Rasigraf, O.; Sapart, C.J.; Jilbert, T.; Jetten, M.S.M.; Röckmann, T.; Van Der Veen, C.; Bânda, N.; Kartal, B.; Ettwig, K.F. Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environ. Sci. Technol. 2015, 49, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Sivan, O.; Adler, M.; Pearson, A.; Gelman, F.; Bar-Or, I.; John, S.C.; Eckert, W. Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol. Oceanogr. 2011, 56, 1536–1544. [Google Scholar] [CrossRef]
- Sivan, O.; Antler, G.; Turchyn, A.V.; Marlow, J.J.; Orphan, V.J. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps. Proc. Natl. Acad. Sci. USA 2014, 111, E4139–E4147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joye, S.B.; Samarkin, V.A.; Bowles, M.W.; Carini, S.A.; Crespo-Medina, M.; Madigan, M.T. Patterns and controls on anaerobic oxidation of methane in extreme environments of varying salinity. Geochim. Cosmochim. Acta Suppl. 2009, 73, A608. [Google Scholar]
- Maignien, L.; Parkes, R.J.; Cragg, B.; Niemann, H.; Knittel, K.; Coulon, S.; Akhmetzhanov, A.; Boon, N. Anaerobic oxidation of methane in hypersaline cold seep sediments. FEMS Microbiol. Ecol. 2013, 83, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Avrahamov, N.; Antler, G.; Yechieli, Y.; Gavrieli, I.; Joye, S.; Saxton, M. Anaerobic oxidation of methane by sulfate in hypersaline groundwater of the Dead Sea aquifer. Geobiology 2014, 12, 511–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Hou, X.; Su, H. Exploration of the relationship between biogas production and microbial community under high salinity conditions. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]



| Reservoir | Depth (cm) | Fraction (%) | Type | ||
|---|---|---|---|---|---|
| Fsa 2–0.063 mm | Fsi 0.063–0.002 mm | Fcl < 0.002 mm | PN-EN ISO 14688–2: 2018–05 | ||
| Maziarnia | 0–5 | 17.49 | 50.00 | 32.51 | loam (Cl) |
| 5–10 | 25.18 | 44.09 | 30.72 | loam (Cl) | |
| 10–15 | 32.18 | 45.82 | 22.00 | clay loam (sasiCl) | |
| Nielisz | 0–5 | 51.27 | 33.28 | 15.44 | clay loam (sasiCl) |
| 5–10 | 34.03 | 45.98 | 19.98 | clay loam (sasiCl) | |
| 10–15 | 21.31 | 56.05 | 22.64 | clay loam (sasiCl) | |
| Rzeszów | 0–5 | 3.50 | 77.35 | 19.15 | silty loam (clSi) |
| 5–10 | 5.23 | 73.15 | 21.61 | silty clay (siCl) | |
| 10–15 | 4.01 | 73.76 | 22.22 | silty clay (siCl) | |
| Reservoir | Depth (cm) | pH | OM (%) | TOCS (%) | HS (mg/g) | Pristane (μg/g) | Crocetane (μg/g) | Squalane (μg/g) | Archaeol (μg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Maziarnia | 0–5 | 7.13 | 4.44 | 1.38 | 14.20 | 0.082 | 0.16 | 0.20 | 1.00 |
| 5–10 | 6.39 | 4.71 | 1.79 | 18.20 | 0.046 | 0.07 | 0.16 | 0.65 | |
| 10–15 | 6.44 | 3.38 | 1.29 | 13.40 | 0.042 | 0.07 | 0.49 | 0.47 | |
| Nielisz | 0–5 | 8.01 | 13.16 | 5.22 | 48.00 | 0.117 | 0.13 | 0.22 | 3.14 |
| 5–10 | 7.99 | 13.18 | 4.94 | 49.60 | 0.101 | 0.13 | 0.19 | 3.48 | |
| 10–15 | 8.03 | 12.47 | 4.97 | 50.50 | 0.075 | 0.10 | 0.17 | 3.34 | |
| Rzeszów | 0–5 | 7.51 | 9.65 | 3.79 | 34.90 | 0.106 | 0.14 | 0.24 | 1.42 |
| 5–10 | 7.58 | 8.18 | 3.84 | 34.70 | 0.072 | 0.09 | 0.27 | 2.44 | |
| 10–15 | 7.71 | 5.99 | 2.66 | 22.90 | 0.057 | 0.07 | 0.68 | 2.06 |
| Reservoir | Depth cm | NO2− mg/dm3 | NO3− mg/dm3 | NH4+ mg/dm3 | SO42− mg/dm3 | Cl− mg/dm3 | Na+ mg/dm3 | K+ mg/dm3 | Mg2+ mg/dm3 | Ca2+ mg/dm3 | TOCw mg/dm3 | IC mg/dm3 | Fe2+ μmol/ dm3 | Fe3+ μmol/ dm3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maziarnia | 0–5 | 0.032 | 0.040 | 5.53 | 14.49 | 17.30 | 16.29 | 5.10 | 4.98 | 50.72 | 20.06 | 64.08 | 69.70 | 896.33 |
| 5–10 | 0.028 | 0.071 | 6.64 | 10.61 | 17.88 | 15.77 | 5.70 | 6.92 | 63.39 | 36.39 | 85.71 | 172.45 | 736.61 | |
| 10–15 | 0.024 | 0.037 | 9.43 | 6.30 | 16.93 | 13.36 | 4.19 | 9.88 | 78.81 | 44.34 | 101.66 | 321.61 | 401.51 | |
| Nielisz | 0–5 | 0.010 | 0.024 | 16.58 | 11.34 | 6.37 | 6.21 | 5.24 | 12.37 | 119.34 | 52.74 | 147.12 | 110.80 | 845.55 |
| 5–10 | 0.015 | 0.029 | 12.70 | 9.70 | 4.81 | 5.59 | 2.63 | 9.73 | 80.62 | 28.54 | 114.30 | 162.17 | 778.95 | |
| 10–15 | 0.018 | 0.036 | 8.07 | 8.40 | 3.20 | 4.99 | 1.35 | 9.00 | 64.79 | 20.64 | 101.50 | 259.78 | 741.85 | |
| Rzeszów | 0–5 | 0.015 | 0.037 | 4.75 | 0.87 | 19.81 | 17.12 | 6.85 | 18.92 | 97.06 | 19.88 | 149.14 | 68.41 | 279.75 |
| 5–10 | 0.018 | 0.052 | 9.61 | 2.56 | 16.70 | 15.77 | 7.46 | 21.79 | 118.59 | 31.44 | 141.44 | 75.93 | 177.15 | |
| 10–15 | 0.015 | 0.020 | 8.04 | 2.37 | 16.12 | 16.43 | 8.64 | 22.67 | 124.42 | 36.56 | 131.80 | 100.52 | 39.45 |
| NO2− | NO3− | NH4+ | SO42− | Cl− | Na+ | K+ | Mg2+ | Ca2+ | TOCw | IC | Fe2+ | Fe3+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOM rate (3 day) | −0.3293 | −0.0577 | −0.0922 | −0.0694 | −0.3295 | −0.3015 | −0.2884 | 0.0333 | −0.0914 | −0.6362 | 0.2740 | −0.3431 | 0.2296 |
| AOM rate (20 day) | −0.5329 | −0.2925 | 0.5989 | 0.2206 | −0.7804 * | −0.8589 ** | −0.6647 | −0.2689 | 0.0055 | 0.2515 | 0.2625 | 0.4285 | 0.4657 |
| AOM rate (40 day) | −0.8646 ** | −0.5910 | 0.3810 | −0.2938 | −0.6325 | −0.5763 | −0.1690 | 0.3968 | 0.4691 | −0.0911 | 0.6676 * | −0.1430 | −0.0673 |
| pH | OM | TOCS | HS | Pristane | Crocetane | Squalane | Archaeol | |
|---|---|---|---|---|---|---|---|---|
| AOM rate (3 day) | 0.5889 | 0.7057 * | 0.6676 * | 0.6840 * | 0.7495 * | 0.6514 | −0.6621 | 0.4943 |
| AOM rate (20 day) | 0.4402 | 0.7513 * | 0.7077 * | 0.7452 * | 0.4804 | 0.1649 | −0.4038 | 0.5778 |
| AOM rate (40 day) | 0.9026 *** | 0.8691 ** | 0.8998 *** | 0.8712 ** | 0.6574 | 0.2801 | −0.0890 | 0.8244 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruca-Rokosz, R.; Szal, D. Isotopic Evidence for Anaerobic Oxidation of Methane in the Freshwater Sediments of Reservoirs: The Impact of Selected Environmental Factors. Water 2022, 14, 3375. https://doi.org/10.3390/w14213375
Gruca-Rokosz R, Szal D. Isotopic Evidence for Anaerobic Oxidation of Methane in the Freshwater Sediments of Reservoirs: The Impact of Selected Environmental Factors. Water. 2022; 14(21):3375. https://doi.org/10.3390/w14213375
Chicago/Turabian StyleGruca-Rokosz, Renata, and Dorota Szal. 2022. "Isotopic Evidence for Anaerobic Oxidation of Methane in the Freshwater Sediments of Reservoirs: The Impact of Selected Environmental Factors" Water 14, no. 21: 3375. https://doi.org/10.3390/w14213375