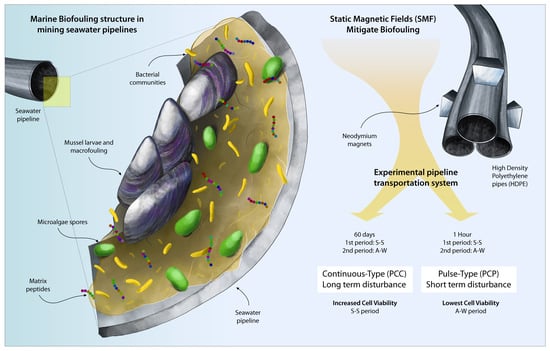
1. Introduction
As a consequence of water scarcity that is currently affecting Chile, as well as many other countries in the world, the use of seawater as a water resource for industrial processes, e.g., mining, has progressively increased in the last decades [
1]. Usually mining areas are far from the coastline, which implies the transportation of seawater for long distance (longer than 100 km) is performed by pipelines [
2,
3]. Marine microorganisms and high salinity in seawater are responsible for the formation of marine life-based biofouling on the inner part of the pipelines, thus, decreasing notably the transport efficiency and, consequently, the industrial systems performance, as well as causing the materials’ property decay and, consequently the shortening of their service life [
4]. A practical and traditional approach to prevent the biofouling is the addition of chemical antifouling compounds to seawater, but this method is not free of environmental concerns [
5].
The richness in nutrients and the high biodiversity that characterize Chile’s marine environment [
6] are the key factors for a rapid biofouling formation on the surface of all types of materials exposed to seawater [
5,
6,
7]. Biofouling is composed of biofilms, i.e., a community of microorganisms attached to a solid surface; [
8,
9] described a high microbial richness (with more than 7300 species revealed from 101 biofilm metagenomes), including sessile bacteria, microalgae including diatoms, microscopic fungi, heterotrophic flagellates and sessile ciliates (heterotrophic protists) [
9,
10]. In general, it has been described that biofouling represents a resistant structure that is composed of layers of biofilm, whose structure is the result of the effects produced by several external agents of climatic or anthropogenic origin [
8], better known with the generic name of disturbances. Disturbances are generally divided in two categories: pulse-type and continuous-type disturbances. A pulse-type disturbance causes a short-term impact that alters the density of communities [
11], e.g., natural disturbances are commonly pulse-type disturbances and are followed by a reorganization phase. Conversely, a continuous-type disturbance causes a long-term impact, sufficient to keep the equilibrium system perturbed for a long time [
11,
12,
13].
Static magnetic field (SMF) represents a sort of disturbance that can affect microbial communities. An SMF is a ubiquitous environmental factor for all living organisms during the evolutionary process. Various studies have proven that bacteria, algae, invertebrates, plants, birds, mice and even humans are capable of detecting the Earth’s magnetic field for orientation in navigation and migration, in case of escape and nest building [
14]. Continuous and pulse exposures of organisms to SMFs of higher intensity than the geomagnetic field has been studied along the years. Several hypotheses have been proposed to explain the interactions between SMFs and biological systems, including magnetic induction, magnetite hypothesis and free radical pair mechanism [
15]. Nevertheless, the exact mechanisms that govern the effect of SMFs on living systems are largely unknown, and there is no single theory that can comprehensively explain the interaction between magnetic field and organisms.
Some studies have described the effect of SMF on microorganisms [
16] through morphological studies using a transmission electron microscope (TEM) and have detected damages to the bacterial cell wall caused by exposure to SMF. In fungi, a mycelial growth inhibition has been observed as a response to 300 mT SMF coupled with morphological and biochemical changes [
17]. The effects of SMF on microorganisms not only depend on the species and their morphology but also on the characteristics of the culture medium where the magnetic field is active [
18]. SMFs in aqueous systems produce changes in the environment properties, thus, modifying the kinetics of physicochemical processes and, consequently, the performance of technological and biological systems that rely on such processes. These variations are generally small but are sufficient to significantly affect biological and industrial systems [
19].
Despite the impacts of SMFs on unicellular microorganisms, there are very few studies describing the effects of SMFs on complex micro communities, such as a marine biofilm, where interspecific biotic, as well abiotic interactions occur among different species of microorganisms and the surrounding environment. Some studies have shown that SMF has an impact on biofilms, however, most of these studies have been performed with single species and in the laboratory. For example, it was determined that in the presence of 200 mT, SMF significantly enhanced biofilm formation and swarming in
Pseudomonas aeruginosa [
20]. In exoelectrogenic biofilms of
Geobacteraceae, it was shown that the use of low-intensity magnetic field (105 and 150 mT) with short-term application increases biofilm conductivity [
21], and Letuta and Tikhonova [
22] demonstrated that
Escherichia coli biofilms are stimulated in the presence of low-intensity SMF (20–35 mT). Therefore, the aim of this research has been to study the composition of the biofilm growing on the inner surface of pipelines, transporting seawater and the effects that the SMFs supplied as continuous- and pulse-type disturbance caused on it, thus, contributing knowledge that will be useful in the future for developing a new antifouling system with a low environmental impact.
2. Materials and Methods
2.1. Experimental Pipeline Transportation System
Eighteen high density polyethylene (HDPE) pipes with a diameter of 1.8 cm and a length of 120 cm were used to transport seawater. The pipeline transportation system was fed with raw seawater collected directly from San Jorge Bay in Antofagasta (latitude 23.70263° S, longitude 70.41984° O) and flowing through the pipeline system continuously and uninterruptedly for 60 observation days during two survey periods, i.e., austral autumn –winter (A–W, June–July) and spring–summer (S–S, November–December) (
Figure 1a). The average flow rate was the same during the two periods, i.e., 0.35 m
3/s. SMF tests were conducted on two separate experimental pipeline transportation systems, in triplicate. Experiments were performed, according to 3 different conditions: (i) pulse-type application of SMF (test Pipeline Pulse type SMF or PPS); (ii) continuous-type application of SMF (test Pipeline Continuous type SMF or PCS); (iii) no application of a static magnetic field as control (test C).
2.2. Tests on Biofouling
SMF tests were conducted under the 3 different conditions, as follows:
In test 1, the experimental pipeline transportation system with seawater flowing continuously was exposed daily to pulse-type SMF (PPS) for 1 h across 60 days.
In test 2, the experimental pipeline transportation system with seawater flowing continuously was exposed daily to continuous-type SMF (PCS) for 1 h across 60 days.
In test 3, (control), the experimental pipeline transportation system with seawater flowing continuously was exposed daily to no SMF (C) across 60 days.
The first SMF tests survey was carried out during A–W period, the second during the S–S period.
2.3. Static Magnetic Field (SMF) Characterization and Control
The magnetic field was produced by neodymium magnets (Nd2Fe14B of grade N33) of moderate intensity, corresponding to 5000 G (Socoter Ltd., Santiago, Chile), coated with Ni/Cu/Ni, having dimensions of 20 × 20 × 20 mm3. The strength of SMF was measured through an AC/DC Gaussmeter model PCE-MFM 3000 (PCE Americas Inc., Southampton, UK). The rare earth (RE) content in the RE–Fe alloy of the magnet, ranges between 30 to 35 Wt%. According to manufacture specifications, the corresponding remaining flux density Br, coercivity values bHc and jHc, and maximum stored energy (BH)max are, respectively 1.164 T, 11.43 kOe and 25.99 kOe, and 33.06 MGOe. The visual control of the SMF was performed by using COMSOL Multiphysics software v5.5. (Comsol Company, Stockholm, Sweden), which is described below.
2.4. Modeling the SMF
The considered geometry consists of three cylinders accounting for the pipelines and 3 sets of 4 cubes which are the magnets. Seawater flows through the pipelines with a mean speed of 1.35 ± 0.4 m/s determined in two steps. (1) Calculating the flow rate (Q) via measuring the time needed to fill a recipient. (2) Calculating the speed using
, where
is the magnitude of the velocity and
is the section of the pipeline. In this case,
= 2.5 cm
2. Then,
= 1.38 m/s. Next step in the analysis is the calculation of the magnetic Reynolds number (
). This number is defined through
[
23], where
is the magnetic diffusivity,
the magnetic permeability of vacuum and
is the fluid conductivity. The latter means that if
, the magnetic flux density
influences the fluid velocity
; however, there is little influence of
on
. It turns out that
, which is ≪ 1. Therefore, we computed the magnetic flux density
in space using the magnetic fields no currents formulation, as the fluid will not affect
. In this case,
is computed through the magnetic scalar potential (
). This formulation was used in the environment of COMSOL Multiphysics v5.5.
The measurements described in 2.3 were used with the aim to find the magnetization of the individual permanent magnets so that the magnetic fields no currents formulation can be defined. The components of
at (x,y,z) away from the permanent magnet of dimensions 2a, 2b and 2c homogeneously magnetized along the z-direction, i.e.
, can be found by evaluating the equations in [
24,
25]; see also [
26] for a complete description. Accordingly, the analytical solution for
in the magnetization axis can be expressed in terms of the magnetic permeability of the ferromagnetic material and geometrical dimensions of the magnet evaluated in a
function. The orthogonal components to the magnetization show a logarithmic form. Simplifying equations, it can be reduced to Equation (1), for which
, W = 2b, D = 2c are the length, width, and depth of a rectangular magnet,
is the remanent flux density,
, and d is the distance measured along the magnetization axis. A measurement at
,
and
, allows finding
or
.
Measuring at the side of each magnet via Gaussmeter, one finds Br to then compute in space using COMSOL Multiphysics, based on the magnetic fields no currents formulation.
2.5. Measurement of Physicochemical Parameters
During the experimental SMF tests, daily measurements of physicochemical parameters of seawater in the effluent, including temperature (°C), pH, salinity (psu), electrical conductivity (S/m) and dissolved oxygen (mg/L), were performed by using a HI 98,194 multi-parameter probe (Hanna Instruments Inc., Woonsocket, RI, USA).
2.6. Biofilm Analysis
At the end of the 60 days of experimentation, the experimental pipes were removed to carry out the biofilm sampling. Once the pipes were removed, they were cut longitudinally, obtaining two parts. The biomass was obtained by scraping the surface. Subsequently, 100 mg of biomass was weighed and placed in test tubes with marine saline solution, followed by periods of sonication for 30 s, alternated by 30 s of ice bath. This process was repeated three times. After sonication, the supernatant of each sample was taken, and serial dilutions were made in marine saline solution.
2.6.1. Cell Viability
The quantification of viable bacteria was performed by means of epifluorescence microscopy using the 10−2 working dilution with the LIVE/DEAD® BacLightTM Bacterial Viability kit, according to the supplier’s instructions (cat. no. L7012, Thermofisher, Waltham, MA, USA), allowing the differentiation of live and dead bacteria present in the biomass to be removed from the experimental pipes.
2.6.2. Metagenomic Analysis
To evaluate the effect of SMF on the biofilm structure, and the microbial communities in the pipes with the treatments and the control condition were characterized after 60 days of operation in the A–W and S–S periods. The extraction of total DNA from biofilm collected from each test was performed by using the phenol:chloroform method [
27]. The gDNA purification was subsequently performed, according to the UltraClean
® 15 DNA Purification Kit by MO BIO protocol (cat. no. 12100-300). The extraction was visualized on a 0.8% agarose gel stained with SYBR Safe DNA gel stain (INVITROGEN, Waltham, MA, USA) by using a 1 kb molecular weight marker (Fermentas, cat. no. SM0311). Total DNA samples were processed by the Laboratorio Genoma Mayor SpA. (Santiago, Chile) for metagenomic analysis by considering 16S rRNA and 18S rRNA marker genes on the Illumina MiSeq platform with 200,000 reads per sample and bioinformatic analysis. The analyzed data correspond to overlapping amplicon sequences obtained on the Illumina platform HiSeq 2 × 250 using the method described by Dr Knight’s lab [
28]. These sequences correspond to 34 samples from the V3–V4 region of the 16S rDNA gene and 30 samples from the V4 region of 18S rDNA gene. The bioinformatic protocol of DADA2 on the R platform for sample analysis was used. The DADA2 package infers sequence variants of the accurate amplicon sequences (ASV) from amplicon sequencing data, replacing the OTU clustering approach. The DADA2 pipeline takes, as input, the
fastq files demultiplexed and generates the sequence variants and their sample abundances after removing errors from substitution and chimera. The taxonomic classification is done through a native implementation of the naive Bayesian Classifier RDP, and the assignment of the 16S rDNA gene sequences is done by exact match.
2.7. Statistical Analysis
To evaluate the significance of variation of the species richness and their relative abundance by varying the SMF tests, the experimental data were processed using the univariate statistical analyses performed in Minitab 17 (Minitab LLC, State College, PA, USA). The analysis of biofilm composition was carried out by using a biological database composed of those OTUs that exhibited a frequency of occurrence over 20% during the period of study. A multivariate canonical redundancy analysis (RDA) was used to evaluate the influence of the environmental variables (temperature, salinity and DO) on the variance of species composition (and their relative abundance) during survey period (A–W, S–S), SMF tests (PPS, PCS) and C conditions. The significance of the ordination axes was evaluated by 500 Monte Carlo permutations [
29]. This analysis was computed CANOCO version 4.5 (Biometrics-Plant Research International, Wageningen, The Netherlands).
4. Discussion
In this study, seawater temperature was influenced by seasonality despite the Humboldt Current influencing the San Jorge Bay in Antofagasta maintains relatively homogeneous the water temperatures throughout the year [
8,
30]. Therefore, during the A–W period, the temperature was 16.2 °C, while during the S–S period, the temperature was a little higher and equal to 19 °C, showing a relative difference up to 20%. The temperature influenced other parameters such as pH, electrical conductivity, salinity and DO, that showed higher values in the A–W period than in S–S (
Table 1): differences between the two survey periods were less than 5% for pH, EC and salinity and approximately 20% for DO. During the same survey period, no significant difference among all environmental parameters was observed by varying the test (i.e., PCS, PPS and C), whereas the SMF significantly affected the cell viability of the biofilm extracted from the inner surface of pipes of the experimental pipeline transportation system (
Figure 4). This result agrees with the three different effects that SMF can have on biofilm, as follows: (i) stimulatory; (ii) inhibitory, or (iii) unobservable [
18] and with the different sensitiveness to SMF that each species can show. For example, Serrano et al. [
31] determined that the enzymatic activity of superoxide dismutase (SOD) for the microalga
Scenedesmus obliquus exhibited an increase in its activity as a response to a continuous-type SMF perturbation, whereas
Nannochloropsis gaditana increased the enzymatic activity when exposed only to the pulse-type SMF perturbation. According to Sergio et al. [
32], disturbances can alter interspecific interactions and produce community-level effects on species, thus, causing a decrease in biomass and cell viability, as well as changes in community structure and biodiversity.
Through the metagenomic studies, the effects of SMFs on the biofilm composition were analyzed. The bacterial composition of the biofilm mainly consisted of OTUs belonging to families of γ-Proteobacteria, α-Proteobacteria, Bacteroidetes and Planctomycetes. These groups of bacteria are the main colonizers of marine biofilm, as highlighted in a study on materials of plastic origin, such as HDPE/LDPE immersed in seawater [
33]. Carson et al. [
34] demonstrated that microorganisms are particularly abundant on polystyrene material by electron microscopy, whereas Oberbeckmann et al. [
35] basing their conclusions on DGGE band variation, suggested that polymer composition not only influences the abundance of attached microorganisms, but also affects the community structure of the biofilm. Results from the present work have highlighted that the variability of OTUs in the control tests depends on seasonality, whereas SMF disturbances did not significantly affect the community behavior. The variability of OTUs in results from tests PPS and PCS during each survey period depends on the sensitivity and tolerance of each species to the magnetic intensity gradient as well as type of perturbation (pulse or continuous).
Arcticiflavibacter spp. and SM1A02 spp. were found only in SMF tests, whereas they were absent in test C. Genera sensitive to SMF disturbance, and they are negatively affected and, therefore, replaced by more tolerant genera, such as the
Arcobacter spp. that is abundant in both PPS and PCS tests (
Figure 4a).
Regarding to the richness and relative abundance of OTUs, no relevant effects among all tests were observed during the A–W period. However, during the S–S period, a higher richness of OTUs was found in test PPS than PCS and C tests. This result is a consequence of the effects of SMF on the species that take part to the biofilm colonization and attachment process, thus, governing the mechanisms responsible for the diversifications in community composition by varying the SMF perturbation. On the contrary, the relative abundance of species during the S–S period was higher in test C than test PPS. Therefore, test PPS was characterized for exhibiting a great richness of species, as well as a low relative abundance. This controversial result is caused by the stress that SMF induces on microbial community that constantly must adapt to and recover from the changes of the environmental conditions.
Canonical redundancy analysis (RDA) showed a significant difference in bacterial composition between SMF tests and C test. The relative abundance of OTUs observed during the S–S period was significantly different between the two group of tests, i.e., under SMF perturbation and control. In both cases, OTUs are positively correlated with temperatures that represents a key factor for biofilm growth, as well as abundance on sediments surface layers, as found by Battin et al. [
36]. Conversely, a significant positive correlation between DO concentration and richness of OTUs was observed during the A–W period. This result is in agreement with those from Kress and Herut [
37]. In test C, the relative abundance of an unique OTU was found, whereas in SMF tests, relative abundances of 17 OTUs were detected, thus, showing that the species richness of these tests is positively correlated with seasonality and DO concentration. Similar results were obtained by Kohno et al. [
38] in studying the effects of SMF perturbations on
Staphylococcus aureus and
Escherichia coli mutants under aerobic and anaerobic conditions, thus, proving that bacterial growth was only inhibited by SMF under anaerobic conditions, whereas no effect was observed under aerobic conditions.
As concerns the composition of eukaryotes, mainly protists and invertebrate larvae were detected, whereas no relevant differences were observed in the composition of OTUs in biofilm between SMF tests and C test. Moreover, the richness of OTUs in test PPS was higher during the A–W period than during the S–S period. Relative abundance was the highest in the PPS test during both survey periods. Finally, RDA showed evident differences in bacteria composition between seasons as well as among the tests, i.e., PPS, PCS and C (
Figure 7b).
As was noticed for bacterial community, the relative abundance of eukaryotic OTUs during the S–S period was correlated with temperature, whereas during the A–W period, it was correlated with DO. Conversely to results from studies on bacteria, the highest richness of eukaryote OTUs was found in test C during the S–S period. According to this result, studies from the literature have showed that among all marine protists, ecologically redundant taxa or dormant species have the potential to increase considerably in number after an environmental perturbation more than other species [
39]. Conversely, many rare taxa may contribute stronger to microbial community dynamics despite their low proportional abundance [
40,
41]. In this study,
Aplanochytrium spp. (belonging to the Stramenopiles), a protist not very abundant in seawater, increased its relative abundance under SMF conditions during the S–S period. It has been described as participating in prey–predator interactions in the grazing food web of the marine ecosystem [
42]. Additionally, evidence have showed that some specialized OTUs belonging mainly to parasites (Cercozoa and MALV) thrive in specific seasonal periods, thus, abruptly increasing their abundance [
39]. In general, Cercozoa both as generalists and as specialists, compose an important group of the protist community, thus, generating connections with a variety of other protists and influencing trophic pathways through parasitism [
41]. Although the dynamics of eukaryotes exhibited in SMF tests, as well as in test C, prove that the diversification of OTUs are related to their tolerance and adaptation skills to SMF, Hadfield et al. [
43] indicate that the colonization of eukaryotic microorganisms or invertebrate larvae also strongly depends on the composition of the primary biofilm.
5. Conclusions
This paper shows the results of the effect of SMF on the marine microbial communities colonizing pipelines for seawater transportation. Depending on the type of perturbation, i.e., pulse-type or continuous-type, and seasonality, the communities forming the biofilm show different dynamics. The cell viability of bacteria in biofilms colonizing the inner surface of the transport pipes was evaluated in the S–S and A–W periods, showing that the number of live bacteria was higher than the number of dead bacteria. During the S–S period, the highest number of live cells (757,780 cells/cm
2) was detected under PCS, while in the A–W period it was detected under PPS (642,843 cells/cm
2), and the lowest viability (349,151 cells/cm
2) was obtained under PCS. This would indicate that during the A–W period, pulse-type perturbation stimulated the microorganisms’ growth, whereas continuous-type perturbation reduced the growth rate. During the S–S period, continuous-type perturbation stimulated the growth of microorganisms while pulse-type perturbation did not generate effects. Moreover, the microorganisms’ community composition was affected by the skill of species to adapt to external perturbations produced by SMF, as well as by seasonal and environmental factors. Metagenomic analysis showed the presence of 819 taxa for bacteria and 441 taxa for eukaryotes in both A–W and S–S sampling periods. In the case of bacteria, only 40 taxa in the A–W period and 38 taxa in the S–S period exhibited a frequency of occurrence greater than 20%, but only 12 OTUs presented relative abundances higher to 5% in S–S (see
Figure 5b). In the case of eukaryotes, the most representative groups were Protists, Nematodes, Arthropods and Ascidians. Among them, only 17 taxa in the A–W period and 18 taxa in the S–S period presented a relative abundance greater than 2%. RDA showed that composition of the bacterial community varied significantly during the annual seasonality, with a significant increase in the relative abundance of OTUs, such as
Candidatus tenderia or
Marinifilum during the S–S period, while in the A–W period,
Articiflavibacter spp. and
Arcobacter spp. were the most abundant. As regards eukaryote, the RDA resulted in the same trend showed by microorganisms’ composition: eukaryotes’ composition varied significantly with annual seasonality, for example,
Acari spp.,
Fragilidium spp. and
Aplanochytrium spp. were only detected during the S–S period, while
Cercozoa were found only during the A–W period.
This research work demonstrates that SMF is capable of directly affecting the composition of biofilms attached to the inner surface of pipelines, transporting seawater and indirectly of the phenomena of biofouling that is due to extracellular polymeric substances (EPS), as the amount and chemical composition of EPS secreted by microorganisms depend on several factors, including microorganism’s strains and abundance. Moreover, results from this study have highlighted that SMF effects on biofilm formation and composition also depend on operating conditions, such as the biodiversity of the geographical region, the position of magnets, the intensity of the SMF, the time of service and the seasonality. Therefore, further studies that are focused on aspects relating to the amount and composition of EPS, as well as the operating conditions of SMF application, are necessary to deeply understand the real potentiality of this treatment to prevent the biofouling formation.