Pore Size in the Removal of Phosphorus and Nitrogen from Poultry Slaughterhouse Wastewater Using Polymeric Nanofiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Study Description, Sampling, Wastewater Characteristics, and Analytical Methods
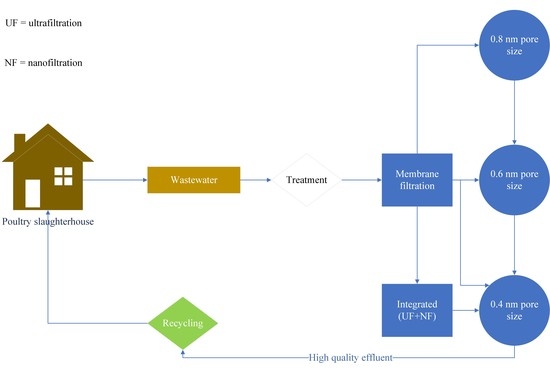
2.2. Experimental Setup and Procedures
2.3. Statistical Methods
2.3.1. Relationship among Parameters
- From 0 to 0.29: Regarded as a weak relationship
- From 0.3 to 0.49: Defined as moderately related parameters
- From 0.5 to 0.69: Defines a strong relationship
- From 0.7 to 1: Defined as a very strong relationship
2.3.2. Data Distribution Analysis
2.3.3. Removal Efficiency Computations
2.3.4. Percent Compliance Computations
2.3.5. Analysis of Variance (ANOVA)
2.3.6. Tukey’s HSD and Scheffé Multiple Comparison Tests
2.3.7. Flux Decline Computations
3. Results and Discussion
3.1. Wastewater Characterization
3.2. Relationships among Parameters in the Raw Wastewater
3.3. Data Distribution in the Raw Wastewater
3.4. Data Distribution in the Treated Effluent Using Nanofiltration
3.5. Removal Efficiencies from the Nanofiltration Systems
3.6. Data Distribution from Integrated Treatment System Effluent
3.7. Removal Efficiencies from the Integrated Treatment System
3.8. Percent Compliance
3.9. Analysis of Variance (ANOVA)
3.10. Tukey’s Honestly Significant Difference
3.11. Scheffé Multiple Comparison
3.12. Flux and Membrane Fouling Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Orians, G.H.; Milewski, A.V. Ecology of Australia: The effects of nutrient-poor soils and intense fires. Biol. Rev. 2007, 82, 393–423. [Google Scholar] [CrossRef]
- Lemley, D.A.; Adams, J.B. Eutrophication. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 86–90. ISBN 9780444641304. [Google Scholar]
- Kateregga, E.; Sterner, T. Indicators for an invasive species: Water hyacinths in Lake Victoria. Ecol. Indic. 2007, 7, 362–370. [Google Scholar] [CrossRef]
- Chambers, P.A.; Vis, C.; Brua, R.B.; Guy, M.; Culp, J.M.; Benoy, G.A. Eutrophication of agricultural streams: Defining nutrient concentrations to protect ecological condition. Water Sci. Technol. 2008, 58, 2203–2210. [Google Scholar] [CrossRef]
- Sato, Y.; Ishihara, M.; Fukuda, K.; Nakamura, S.; Murakami, K.; Fujita, M.; Yokoe, H. Behavior of Nitrate-Nitrogen and Nitrite-Nitrogen in Drinking Water. Biocontrol Sci. 2018, 23, 139–143. [Google Scholar] [CrossRef]
- Jiao, X.; Maimaitiyiming, A.; Salahou, M.; Liu, K.; Guo, W. Impact of Groundwater Level on Nitrate Nitrogen Accumulation in the Vadose Zone Beneath a Cotton Field. Water 2017, 9, 171. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Shao, Y.; Chen, J. Thermodynamic analysis of a new ammonia-water power cycle. Energy Rep. 2020, 6, 567–573. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef]
- Avery, A.A. Infantile methemoglobinemia: Reexamining the role of drinking water nitrates. Environ. Health Perspect. 1999, 107, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D. The Blue Baby Syndrome. Resonance 2003, 8, 20–30. [Google Scholar] [CrossRef]
- Knobeloch, L.; Salna, B.; Hogan, A.; Postle, J.; Anderson, H. Blue babies and nitrate-contaminated well water. Environ. Health Perspect. 2000, 108, 675–678. [Google Scholar] [CrossRef]
- Khosravi, A.; Zarei, M.; Ronaghi, A. Influence of Biofertilizers and Phosphate Sources on the Phosphorus Uptake of Lettuce and Chemical Forms of Phosphorus in Soil. Commun. Soil Sci. Plant Anal. 2017, 48, 2701–2714. [Google Scholar] [CrossRef]
- Kesari, K.K.; Verma, H.N.; Behari, J. Physical methods in wastewater treatment. Int. J. Environ. Technol. Manag. 2011, 14, 43. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Mkilima, T.; Baituk, G.; Beisembayeva, K.; Meirbekov, A.; Kakabayev, A.; Adilbektegi, G.; Tleukulov, A.; Tazhkenova, G. Treatment of waste stabilization pond effluent using natural zeolite for irrigation potential. PLoS ONE 2022, 17, e0259614. [Google Scholar] [CrossRef] [PubMed]
- Kuntubek, A.; Kinayat, N.; Meiramkulova, K.; Poulopoulos, S.G.; Bear, J.C.; Inglezakis, V.J. Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites. Processes 2020, 8, 471. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Jakupova, Z.; Orynbekov, D.; Tashenov, E.; Kydyrbekova, A.; Mkilima, T.; Inglezakis, V.J. Evaluation of Electrochemical Methods for Poultry Slaughterhouse Wastewater Treatment. Sustainability 2020, 12, 5110. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Bazarbayeva, T.; Orynbassar, R.; Tleukulov, A.; Madina, N.; Mashan, T.; Dariya, A.; Apendina, A.; Nurmukhanbetova, N. Assessing the Influence of Electrode Polarity on the Treatment of Poultry Slaughterhouse Wastewater. Molecules 2022, 27, 1014. [Google Scholar] [CrossRef] [PubMed]
- Delforno, T.P.; Lacerda Júnior, G.V.; Noronha, M.F.; Sakamoto, I.K.; Varesche, M.B.A.; Oliveira, V.M. Microbial diversity of a full-scale UASB reactor applied to poultry slaughterhouse wastewater treatment: Integration of 16S rRNA gene amplicon and shotgun metagenomic sequencing. Microbiologyopen 2017, 6, e00443. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Devrishov, D.; Zhumagulov, M.; Arystanova, S.; Karagoishin, Z.; Marzanova, S.; Kydyrbekova, A.; Mkilima, T.; Li, J. Performance of an Integrated Membrane Process with Electrochemical Pre-Treatment on Poultry Slaughterhouse Wastewater Purification. Membranes 2020, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Meiramkulova, K.; Devrishov, D.; Marzanov, N.; Marzanova, S.; Kydyrbekova, A.; Uryumtseva, T.; Tastanova, L.; Mkilima, T. Performance of Graphite and Titanium as Cathode Electrode Materials on Poultry Slaughterhouse Wastewater Treatment. Materials 2020, 13, 4489. [Google Scholar] [CrossRef] [PubMed]
- Makhatova, A.; Mazhit, B.; Sarbassov, Y.; Meiramkulova, K.; Inglezakis, V.J.; Poulopoulos, S.G. Effective photochemical treatment of a municipal solid waste landfill leachate. PLoS ONE 2020, 15, e0239433. [Google Scholar] [CrossRef] [PubMed]
- Del Nery, V.; Damianovic, M.H.Z.; Pozzi, E.; de Nardi, I.R.; Caldas, V.E.A.; Pires, E.C. Long-term performance and operational strategies of a poultry slaughterhouse waste stabilization pond system in a tropical climate. Resour. Conserv. Recycl. 2013, 71, 7–14. [Google Scholar] [CrossRef]
- Baker, B.R.; Mohamed, R.; Al-Gheethi, A.; Aziz, H.A. Advanced technologies for poultry slaughterhouse wastewater treatment: A systematic review. J. Dispers. Sci. Technol. 2021, 42, 880–899. [Google Scholar] [CrossRef]
- Żyłka, R.; Dąbrowski, W.; Gogina, E.; Yancen, O. Trickling Filter for High Efficiency Treatment of Dairy Sewage. J. Ecol. Eng. 2018, 19, 269–275. [Google Scholar] [CrossRef]
- Godini, K.; Azarian, G.; Kimiaei, A.; Dragoi, E.N.; Curteanu, S. Modeling of a real industrial wastewater treatment plant based on aerated lagoon using a neuro-evolutive technique. Process Saf. Environ. Prot. 2021, 148, 114–124. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Mkilima, T.; Meiramkulova, K.; Nurbala, U.; Zandybay, A.; Khusainov, M.; Nurmukhanbetova, N.; Tastanova, L.; Mashan, T.; Meirbekov, A. Investigating the Influence of Column Depth on the Treatment of Textile Wastewater Using Natural Zeolite. Molecules 2021, 26, 7030. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wei, D.; Zhang, S.; Ren, Q.; Shi, J.; Liu, L. Removal of antibiotic resistance genes from swine wastewater by membrane filtration treatment. Ecotoxicol. Environ. Saf. 2021, 210, 111885. [Google Scholar] [CrossRef]
- Thaçi, B.S.; Gashi, S.T. Reverse osmosis removal of heavy metals from wastewater effluents using biowaste materials pretreatment. Pol. J. Environ. Stud. 2019. [Google Scholar] [CrossRef]
- Kyllönen, H.; Heikkinen, J.; Ceras, J.; Fernandez, C.; Porc, O.; Grönroos, A. Membrane-based conceptual design of reuse water production from candy factory wastewater. Water Sci. Technol. 2021, 84, 1389–1402. [Google Scholar] [CrossRef]
- Afanga, H.; Zazou, H.; Titchou, F.E.; Rakhila, Y.; Akbour, R.A.; Elmchaouri, A.; Ghanbaja, J.; Hamdani, M. Integrated electrochemical processes for textile industry wastewater treatment: System performances and sludge settling characteristics. Sustain. Environ. Res. 2020, 30, 2. [Google Scholar] [CrossRef] [Green Version]
- Aziz, H.A.; Puat, N.N.A.; Alazaiza, M.Y.D.; Hung, Y.T. Poultry slaughterhouse wastewater treatment using submerged fibers in an attached growth sequential batch reactor. Int. J. Environ. Res. Public Health 2018, 15, 1734. [Google Scholar] [CrossRef] [PubMed]
- Meiramkulova, K.; Orynbekov, D.; Saspugayeva, G.; Aubakirova, K.; Arystanova, S.; Kydyrbekova, A.; Tashenov, E.; Nurlan, K.; Mkilima, T. The Effect of Mixing Ratios on the Performance of an Integrated Poultry Slaughterhouse Wastewater Treatment Plant for a Recyclable High-Quality Effluent. Sustainability 2020, 12, 6097. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Makhatova, A.; Meiramkulova, K.; Poulopoulos, S.G. Treatment of a poultry slaughterhouse wastewater using advanced oxidation processes. J. Water Process Eng. 2022, 47, 102694. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Zorpas, A.A.; Orynbekov, D.; Zhumagulov, M.; Saspugayeva, G.; Kydyrbekova, A.; Mkilima, T.; Inglezakis, V.J. The Effect of Scale on the Performance of an Integrated Poultry Slaughterhouse Wastewater Treatment Process. Sustainability 2020, 12, 4679. [Google Scholar] [CrossRef]
- Frascari, D.; Zanaroli, G.; Motaleb, M.A.; Annen, G.; Belguith, K.; Borin, S.; Choukr-Allah, R.; Gibert, C.; Jaouani, A.; Kalogerakis, N.; et al. Integrated technological and management solutions for wastewater treatment and efficient agricultural reuse in Egypt, Morocco, and Tunisia. Integr. Environ. Assess. Manag. 2018, 14, 447–462. [Google Scholar] [CrossRef]
- Hoh, D.; Watson, S.; Kan, E. Algal biofilm reactors for integrated wastewater treatment and biofuel production: A review. Chem. Eng. J. 2016, 287, 466–473. [Google Scholar] [CrossRef]
- Hach Company. Standard Solutions: Chemicals and Reagents; Hach Company: Frederick, MD, USA, 2007; Volume 2549. [Google Scholar]
- Gönder, Z.B.; Kaya, Y.; Vergili, I.; Barlas, H. Optimization of filtration conditions for CIP wastewater treatment by nanofiltration process using Taguchi approach. Sep. Purif. Technol. 2010, 70, 265–273. [Google Scholar] [CrossRef]
- Lee, Y.; Clark, M.M. Modeling of flux decline during crossflow ultrafiltration of colloidal suspensions. J. Memb. Sci. 1998, 149, 181–202. [Google Scholar] [CrossRef]
- Baker, J.; Battye, W.H.; Robarge, W.; Pal Arya, S.; Aneja, V.P. Modeling and measurements of ammonia from poultry operations: Their emissions, transport, and deposition in the Chesapeake Bay. Sci. Total Environ. 2020, 706, 135290. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Xiao, X.-C.; Wang, H.-Z.; Li, Y.; Yu, Q.; Liang, X.-M.; Feng, W.-S.; Shao, J.-C.; Rybicki, M.; Jungmann, D.; et al. Effects of high ammonia concentrations on three cyprinid fish: Acute and whole-ecosystem chronic tests. Sci. Total Environ. 2017, 598, 900–909. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Aquatic Life Criteria—Ammonia; United States Environmental Protection Agency: Washington, DC, USA, 2015.
- Gurung, D.P.; Githinji, L.J.M.; Ankumah, R.O. Assessing the Nitrogen and Phosphorus Loading in the Alabama (USA) River Basin Using PLOAD Model. Air Soil Water Res. 2013, 6, ASWR.S10548. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Tu, B. Immobilization of ammonia-oxidizing bacteria by polyvinyl alcohol and sodium alginate. Braz. J. Microbiol. 2017, 48, 515–521. [Google Scholar] [CrossRef]
- Tiso, M.; Schechter, A.N. Nitrate Reduction to Nitrite, Nitric Oxide and Ammonia by Gut Bacteria under Physiological Conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar] [CrossRef]
- Larsen, R.D. Box-and-whisker plots. J. Chem. Educ. 1985, 62, 302. [Google Scholar] [CrossRef]
- Liu, M.; Lü, Z.; Chen, Z.; Yu, S.; Gao, C. Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 2011, 281, 372–378. [Google Scholar] [CrossRef]
- Amar, N.B.; Kechaou, N.; Palmeri, J.; Deratani, A.; Sghaier, A. Comparison of tertiary treatment by nanofiltration and reverse osmosis for water reuse in denim textile industry. J. Hazard. Mater. 2009, 170, 111–117. [Google Scholar] [CrossRef]
- Monnot, M.; Nguyên, H.T.K.; Laborie, S.; Cabassud, C. Seawater reverse osmosis desalination plant at community-scale: Role of an innovative pretreatment on process performances and intensification. Chem. Eng. Process. Process. Intensif. 2017, 113, 42–55. [Google Scholar] [CrossRef]
- Mahat, S.B.; Omar, R.; Lee, J.L.; Mohd Idris, A.I.; Che Man, H.; Mustapa Kamal, S.M.; Idris, A. Effect of pore size of monofilament woven filter cloth as supporting material for dynamic membrane filtration on performance using aerobic membrane bioreactor technology. Asia-Pac. J. Chem. Eng. 2020, 15, e2453. [Google Scholar] [CrossRef]
- Hassan, M.L.; Fadel, S.M.; Abouzeid, R.E.; Abou Elseoud, W.S.; Hassan, E.A.; Berglund, L.; Oksman, K. Water purification ultrafiltration membranes using nanofibers from unbleached and bleached rice straw. Sci. Rep. 2020, 10, 11278. [Google Scholar] [CrossRef]
- Miyamoto, M.; Nakashimada, Y.; Uemiya, S. Membrane reactors for biogas production and processing. In Membrane Reactors for Energy Applications and Basic Chemical Production; Elsevier: Amsterdam, The Netherlands, 2015; pp. 345–365. ISBN 9781782422273. [Google Scholar]
- Lin, H.; Yu, G.; Shen, L.; Li, R.; Xu, Y. Advanced membrane bioreactor fouling control and prevention strategies. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–224. ISBN 9780128198094. [Google Scholar]
- Tibi, F.; Park, S.-J.; Kim, J. Improvement of Membrane Distillation Using PVDF Membrane Incorporated with TiO2 Modified by Silane and Optimization of Fabricating Conditions. Membranes 2021, 11, 95. [Google Scholar] [CrossRef]
- Zhao, F.; Han, X.; Shao, Z.; Li, Z.; Li, Z.; Chen, D. Effects of different pore sizes on membrane fouling and their performance in algae harvesting. J. Memb. Sci. 2022, 641, 119916. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Liao, C.-Y.; Tung, K.-L. Effect of membrane pore size on the particle fouling in membrane filtration. Desalination 2008, 234, 16–23. [Google Scholar] [CrossRef]
- Mora, F.; Pérez, K.; Quezada, C.; Herrera, C.; Cassano, A.; Ruby-Figueroa, R. Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration. Membranes 2019, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Koseoglu, H.; Guler, E.; Harman, B.I.; Gonulsuz, E. Water Flux and Reverse Salt Flux. In Membrane-Based Salinity Gradient Processes for Water Treatment and Power Generation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 57–86. ISBN 9780444639615. [Google Scholar]
- Sadr, S.M.K.; Saroj, D.P. Membrane technologies for municipal wastewater treatment. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 443–463. ISBN 9781782421269. [Google Scholar]









| Parameter | Min | Max | Median | AM | SD | Guideline | Agency |
|---|---|---|---|---|---|---|---|
| Ammonia | 44.99 | 104 | 68.525 | 71.51 | 22.674 | 32.5 | US EPA |
| Total phosphorous | 45 | 189.44 | 124.08 | 120.65 | 53.401 | 10 | US EPA |
| Nitrites | 45.3 | 80 | 64.285 | 63.4675 | 12.350 | 1 | US EPA |
| Nitrate | 25.8 | 178.4 | 99.95 | 103.41 | 54.181 | 0.1 | WHO |
| Parameter | Unit | Value |
|---|---|---|
| Pore size | nm | 0.4, 0.6, and 0.8 |
| Pump supply voltage | V | 36 |
| Pump power | kW | 0.3–0.5 |
| Ammonia | Total Phosphorous | Nitrites | Nitrate | |
|---|---|---|---|---|
| Ammonia | 1 | |||
| Total phosphorous | 0.13 | 1 | ||
| Nitrites | 0.94 | 0.41 | 1 | |
| Nitrate | 0.87 | 0.59 | 0.97 | 1 |
| Parameter | Min | Max | Median | Mean | STD | Removal Efficiency (%) |
|---|---|---|---|---|---|---|
| Ammonia | 1.21 | 2.24 | 1.45 | 1.588 | 0.392 | 97.78 |
| Total phosphorous | 0.001 | 0.03 | 0.02 | 0.018 | 0.010 | 98.33 |
| Nitrites | 0.001 | 0.005 | 0.003 | 0.003 | 0.002 | 98.42 |
| Nitrate | 0.2 | 0.5 | 0.25 | 0.3 | 0.122 | 99.71 |
| Parameter | Raw Wastewater (%) | 0.8 (%) | 0.6 (%) | 0.4 (%) | Integrated (%) |
|---|---|---|---|---|---|
| Ammonia | −120.03 | 0.05 | 27.74 | 50.418 | 95.12 |
| Total phosphorous | −1106.5 | −105.18 | −121.4 | −23.15 | 99.82 |
| Nitrites | −6246.75 | −985.75 | −935.75 | −767.667 | 99.70 |
| Nitrate | −103,310 | −15,675 | −13,025 | −9720 | 67.31 |
| Parameter | F Crit | p-Value | Status (Is p-Value < 0.05?) |
|---|---|---|---|
| Ammonium | 3.708 | 0.000138 | TRUE |
| Phosphates | 3.411 | 1.08 × 10−6 | TRUE |
| Nitrites | 3.411 | 8.13× 10−5 | TRUE |
| Nitrate | 3.587 | 0.001481 | TRUE |
| Treatment Pairs | Tukey’s HSD Q Statistic | Tukey’s HSD p-Value | Tukey’s HSD Inference |
|---|---|---|---|
| 0.8 vs. 0.6 | 1.3226 | 0.770407 | insignificant |
| 0.8 vs. 0.4 | 8.8967 | 0.001005 | ** p < 0.01 |
| 0.8 vs. integrated | 7.894 | 0.001116 | ** p < 0.01 |
| 0.6 vs. 0.4 | 7.4181 | 0.001778 | ** p < 0.01 |
| 0.6 vs. integrated | 6.5714 | 0.004229 | ** p < 0.01 |
| 0.4 vs. integrated | 0.071 | 0.899995 | insignificant |
| Treatment Pairs | Tukey’s HSD Q Statistic | Tukey’s HSD p-Value | Tukey’s HSD Inference |
|---|---|---|---|
| 0.8 vs. 0.6 | 6.1333 | 0.003926 | ** p < 0.01 |
| 0.8 vs. 0.4 | 12.4801 | 0.001005 | ** p < 0.01 |
| 0.8 vs. integrated | 13.1714 | 0.001005 | ** p < 0.01 |
| 0.6 vs. 0.4 | 5.7614 | 0.00631 | ** p < 0.01 |
| 0.6 vs. integrated | 7.493 | 0.001005 | ** p < 0.01 |
| 0.4 vs. integrated | 2.834 | 0.236134 | insignificant |
| Treatment Pairs | Scheffé TT-Statistic | Scheffé p-Value | Scheffé Inference |
|---|---|---|---|
| 0.8 vs. 0.6 | 0.9352 | 0.830639 | insignificant |
| 0.8 vs. 0.4 | 6.2909 | 0.000824 | ** p < 0.01 |
| 0.8 vs. integrated | 5.5819 | 0.002047 | ** p < 0.01 |
| 0.6 vs. 0.4 | 5.2454 | 0.003213 | ** p < 0.01 |
| 0.6 vs. integrated | 4.6467 | 0.007378 | ** p < 0.01 |
| 0.4 vs. integrated | 0.0502 | 0.999964 | insignificant |
| Treatment Pairs | Scheffé TT-Statistic | Scheffé p-Value | Scheffé Inference |
|---|---|---|---|
| 0.8 vs. 0.6 | 4.3369 | 0.007271 | ** p < 0.01 |
| 0.8 vs. 0.4 | 8.8248 | 9.22 × 10−6 | ** p < 0.01 |
| 0.8 vs. integrated | 9.3136 | 5.07 × 10−6 | ** p < 0.01 |
| 0.6 vs. 0.4 | 4.0739 | 0.011374 | * p < 0.05 |
| 0.6 vs. integrated | 5.2984 | 0.001464 | ** p < 0.01 |
| 0.4 vs. integrated | 2.0039 | 0.304666 | insignificant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkilima, T.; Bazarbayeva, T.; Assel, K.; Nurmukhanbetova, N.N.; Ostretsova, I.B.; Khamitova, A.S.; Makhanova, S.; Sergazina, S. Pore Size in the Removal of Phosphorus and Nitrogen from Poultry Slaughterhouse Wastewater Using Polymeric Nanofiltration Membranes. Water 2022, 14, 2929. https://doi.org/10.3390/w14182929
Mkilima T, Bazarbayeva T, Assel K, Nurmukhanbetova NN, Ostretsova IB, Khamitova AS, Makhanova S, Sergazina S. Pore Size in the Removal of Phosphorus and Nitrogen from Poultry Slaughterhouse Wastewater Using Polymeric Nanofiltration Membranes. Water. 2022; 14(18):2929. https://doi.org/10.3390/w14182929
Chicago/Turabian StyleMkilima, Timoth, Tursynkul Bazarbayeva, Kydyrbekova Assel, Nurgul Nurkenovna Nurmukhanbetova, Idiya Bolatovna Ostretsova, Aina Sultanseitovna Khamitova, Saule Makhanova, and Samal Sergazina. 2022. "Pore Size in the Removal of Phosphorus and Nitrogen from Poultry Slaughterhouse Wastewater Using Polymeric Nanofiltration Membranes" Water 14, no. 18: 2929. https://doi.org/10.3390/w14182929