Simultaneous Removal of CODMn and Ammonium from Water by Potassium Ferrate-Enhanced Iron-Manganese Co-Oxide Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Water Quality and the Pilot-Scale System
2.2. Pollutant Removal Experiments
2.2.1. K2FeO4-Enhanced Filtration to Remove CODMn
2.2.2. Simultaneous Removal of CODMn and NH4+
2.3. Influential Factors on the Removal of CODMn
2.4. Analytic Methods and Characterization Methods
3. Results
3.1. The Removal of CODMn and NH4+
3.1.1. K2FeO4-Enhanced Filtration to Remove CODMn
3.1.2. Simultaneous Removal of CODMn and NH4+
3.2. Influential Factors on the Removal of CODMn
3.2.1. Effect of Filtration Rate
3.2.2. Effect of pH
3.2.3. Effect of Temperature
3.3. Surface Property Variation of MeOx
3.3.1. The Morphology of the MeOx
3.3.2. Characterization of EDS
3.3.3. XPS of the Oxide Film
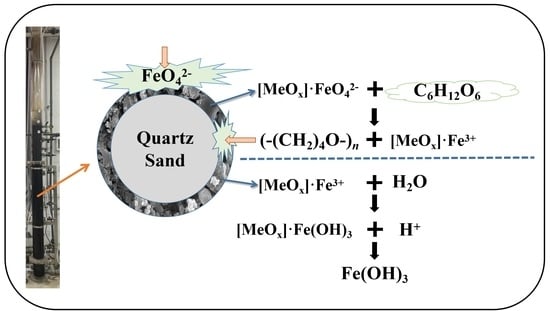
3.4. Proposed Mechanism for CODMn Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, Q.; Fang, N.; Liu, L.; Yang, W.; Zhang, L.; Wang, Y. Effects of land use, topography, climate and socio-economic factors on geographical variation pattern of inland surface water quality in China. PLoS ONE 2019, 14, e0217840. [Google Scholar]
- Yu, Y.; Zhang, C.; Ding, W.; Zhang, Z.; Wang, G.G.X. Determining the performance for an integrated process of COD removal and CO2 capture. J. Clean. Prod. 2020, 275, 122845. [Google Scholar]
- Dos Santos, N.O.; Teixeira, L.A.; Zhou, Q.; Burke, G.; Campos, L.C. Fenton pre-oxidation of natural organic matter in drinking water treatment through the application of iron nails. Environ. Technol. 2021, 43, 2590–2603. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar]
- Yadu, A.; Sahariah, B.P.; Anandkumar, J. Influence of COD/ammonia ratio on simultaneous removal of NH4+-N and COD in surface water using moving bed batch reactor. J. Water Process Eng. 2018, 22, 66–72. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, F.; Liu, C.; Zhang, Y.; Wang, Y.; Song, Z.; Kumirska, J.; Sun, D. Cyanobacteria derived taste and odor characteristics in various lakes in China: Songhua Lake, Chaohu Lake and Taihu Lake. Ecotoxicol. Environ. Saf. 2019, 181, 499–507. [Google Scholar] [CrossRef]
- Tabassum, S. A combined treatment method of novel Mass Bio System and ion exchange for the removal of ammonia nitrogen from micro-polluted water bodies. Chem. Eng. J. 2019, 378, 122217. [Google Scholar] [CrossRef]
- Dos Santos, P.R.; Daniel, L.A. A review: Organic matter and ammonia removal by biological activated carbon filtration for water and wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 591–606. [Google Scholar] [CrossRef]
- Szatyłowicz, E.; Skoczko, I. Studies on the efficiency of groundwater treatment process with adsorption on activated alumina. J. Ecol. Eng. 2018, 18, 211–218. [Google Scholar] [CrossRef]
- Xu, Q.; Li, W.; Ma, L.; Cao, D.; Owens, G.; Chen, Z. Simultaneous removal of ammonia and phosphate using green synthesized iron oxide nanoparticles dispersed onto zeolite. Sci. Total Environ. 2020, 703, 135002. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C. Experimental Study on UF-NF filtration purification of pipe drinking water. J. Phys. Conf. Ser. 2019, 1176, 062021. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, L.; Tang, X.; Huang, Q.; Xie, B.; Wang, T.; Wang, J.; Li, G.; Liang, H. Coupling continuous sand filtration to ultrafiltration for drinking water treatment: Improved performance and membrane fouling control. J. Membr. Sci. 2018, 567, 18–27. [Google Scholar] [CrossRef]
- Xu, K.; Wang, J.; Li, J.; Wang, Z.; Lin, Z. Attapulgite suspension filter material for biological aerated filter to remove CODMn and ammonia nitrogen in micro-polluted drinking water source. Environ. Prot. Eng. 2020, 46, 21–40. [Google Scholar]
- Liu, J.; Xie, S.; Cheng, C.; Lou, J.; Li, S. Effect on bed material heights to the performance of ZCBAF in the treatment of micro-polluted raw water. Appl. Mech. Mater. 2012, 209–211, 2053–2057. [Google Scholar] [CrossRef]
- Terry, L.G.; Summers, R.S. Biodegradable organic matter and rapid-rate biofilter performance: A review. Water Res. 2018, 128, 234–245. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, S.; Huang, T.; Li, Y. Arsenite removal from groundwater by iron–manganese oxides filter media: Behavior and mechanism. Water Environ. Res. 2019, 91, 536–545. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, T.; Wen, G.; Cao, X. The simultaneous removal of ammonium and manganese from groundwater by iron-manganese co-oxide filter film: The role of chemical catalytic oxidation for ammonium removal. Chem. Eng. J. 2017, 308, 322–329. [Google Scholar] [CrossRef]
- Sharma, V.K.; Zboril, R.; Varma, R.S. Ferrates: Greener oxidants with multimodal action in water treatment technologies. Acc. Chem. Res. 2015, 48, 182–191. [Google Scholar] [CrossRef]
- Tran, T.K.; Nguyen, D.H.C.; Hoang, G.P.; Nguyen, T.T.; Nguyen, N.H. Application of ferrate as coagulant and oxidant alternative for purifying Saigon river water. VNU J. Sci. Earth Environ. Sci. 2020, 36, 1–7. [Google Scholar]
- State Environmental Protection Administration. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 224–226. [Google Scholar]
- Guo, Y.; Ma, B.; Huang, J.; Yang, J.; Zhang, R. The simultaneous removal of bisphenol A, manganese and ammonium from groundwater by MeOx: The role of chemical catalytic oxidation for bisphenol A. Water Supply 2022, 22, 2106–2116. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Zhang, H. Oxidative degradation of emerging organic contaminants in aqueous solution by high valent manganese and iron. Prog. Chem. 2021, 33, 1201–1211. [Google Scholar]
- Cheng, Y.; Zhang, S.; Huang, T.; Hu, F.; Gao, M.; Niu, X. Effect of alkalinity on catalytic activity of iron-manganese co-oxide in removing ammonium and manganese: Performance and mechanism. Int. J. Environ. Res. Public Health 2020, 17, 784. [Google Scholar] [CrossRef] [PubMed]
- López, G.P.; Castner, D.G.; Ratner, B.D. XPS O1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf. Interface Anal. 1991, 17, 267–272. [Google Scholar] [CrossRef]
- Hercule, B.R. Surface spectroscopic characterization of Mn/AI2O3 catalysts. J. Chem. Phys. 1984, 88, 4922–4929. [Google Scholar]
- Oku, M.; Hirokawa, K. X-ray photoelectron spectroscopy of Co3O4, Fe3O4, Mn3O4, and related compounds. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 475–481. [Google Scholar] [CrossRef]
- Audi, A.A.; Sherwood, P.M.A. Valence-band X-ray photoelectron spectroscopic studies of manganese and its oxides interpreted by cluster and band structure calculations. Surf. Interface Anal. 2002, 33, 274–282. [Google Scholar] [CrossRef]
- Oku, M.; Hirokawa, K.; Ikeda, S. X-ray photoelectron spectroscopy of manganese–oxygen systems. J. Electron Spectrosc. Relat. Phenom. 1975, 7, 465–473. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Chen, X.; Yang, J.; Huang, J.; Huang, T. Kinetics and mechanism of Mn2+ removal from groundwater using iron-manganese co-oxide filter film. Water Supply 2019, 19, 1711–1717. [Google Scholar] [CrossRef]











| Index | Unit | Value | Surface Water Quality Standard Class III (GBT3838-2002) |
|---|---|---|---|
| Ammonium | mg·L−1 | 0–0.2 | ≤1.0 |
| CODMn | mg·L−1 | 0.87–2.10 | ≤6.0 |
| Nitrate | mg·L−1 | 3.8–4.3 | ≤10.0 |
| Manganese | mg·L−1 | 0–0.05 | ≤0.1 |
| pH | - | 7.5–8.0 | 6.0~9.0 |
| Iron | mg·L−1 | 0.051–0.062 | ≤0.3 |
| Temperature | °C | 14.9–26.5 | - |
| Dissolved oxygen (DO) | mg·L−1 | 8.0–9.5 | ≥5.0 |
| Column | Filter Material | CODMn | K2FeO4 |
|---|---|---|---|
| I | virgin quartz sands | 20.0 mg/L | 0.1 mg/L |
| II | mature sands | 20.0 mg/L | 0.1 mg/L |
| III | mature sands | 20.0 mg/L | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Ma, B.; Yuan, S.; Zhang, Y.; Yang, J.; Zhang, R.; Liu, L. Simultaneous Removal of CODMn and Ammonium from Water by Potassium Ferrate-Enhanced Iron-Manganese Co-Oxide Film. Water 2022, 14, 2651. https://doi.org/10.3390/w14172651
Guo Y, Ma B, Yuan S, Zhang Y, Yang J, Zhang R, Liu L. Simultaneous Removal of CODMn and Ammonium from Water by Potassium Ferrate-Enhanced Iron-Manganese Co-Oxide Film. Water. 2022; 14(17):2651. https://doi.org/10.3390/w14172651
Chicago/Turabian StyleGuo, Yingming, Ben Ma, Shengchen Yuan, Yuhong Zhang, Jing Yang, Ruifeng Zhang, and Longlong Liu. 2022. "Simultaneous Removal of CODMn and Ammonium from Water by Potassium Ferrate-Enhanced Iron-Manganese Co-Oxide Film" Water 14, no. 17: 2651. https://doi.org/10.3390/w14172651