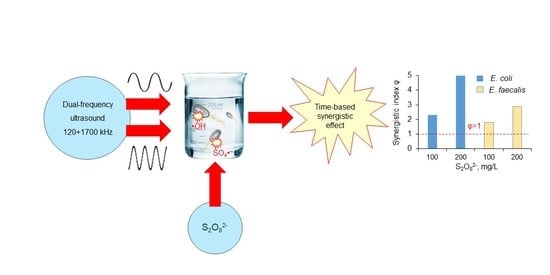
Dual-Frequency Ultrasonic Inactivation of Escherichia coli and Enterococcus faecalis Using Persulfate: A Synergistic Effect
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Drinking Water, Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 13 July 2022).
- Joyce, E.M.; Mason, T.J. Sonication used as a biocide. A review: Ultrasound a greener alternative to chemical biocides? Chim. Oggi Chem. Today 2008, 26, 22–26. [Google Scholar]
- Phull, S.S.; Newman, A.P.; Lorimer, J.P.; Pollet, B.; Mason, T.J. The development and evaluation of ultrasound in the biocidal treatment of water. Ultrason. Sonochem. 1997, 4, 157–164. [Google Scholar] [CrossRef]
- Jyoti, K.K.; Pandit, A.B. Water disinfection by acoustic and hydrodynamic cavitation. Biochem. Eng. J. 2001, 7, 201–212. [Google Scholar] [CrossRef]
- Ananta, E.; Voigt, D.; Zenker, M.; Heinz, V.; Knorr, D. Cellular injuries upon exposure of Escherichia coli and Lactobacillus rhamnosus to high-intensity ultrasound. J. Appl. Microbiol. 2005, 99, 271–278. [Google Scholar] [CrossRef]
- Gao, S.; Hemar, Y.; Ashokkumar, M.; Paturel, S.; Lewis, G.D. Inactivation of bacteria and yeast using high frequency ultrasound treatment. Water Res. 2014, 60, 93–104. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Dual-frequency ultrasound: Strengths and shortcomings to water treatment and disinfection. Water Res. 2020, 182, 116016. [Google Scholar] [CrossRef]
- Wu, X.; Mason, T.J. Evaluation of power ultrasonic effects on algae cells at a small pilot scale. Water 2017, 9, 470. [Google Scholar] [CrossRef]
- Zou, H.; Wang, L. The disinfection effect of a novel continuous-flow water sterilizing system coupling dual-frequency ultrasound with sodium hypochlorite in pilot scale. Ultrason. Sonochem. 2017, 36, 246–252. [Google Scholar] [CrossRef]
- Zou, H.; Tang, H. Comparison of different bacteria inactivation by a novel continuous-flow ultrasound/chlorination water treatment system in a pilot scale. Water 2019, 11, 258. [Google Scholar] [CrossRef]
- Rengeng, L.; Qianyu, Z.; Yuehong, L.; Zhongzhong, P.; Libo, L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagn. Photodyn. Ther. 2017, 19, 159–166. [Google Scholar] [CrossRef]
- Tabatabaei, Z.S.; Rajabi, O.; Nassirli, H.; Noghreiyan, A.V.; Sazgarnia, A. A comparative study on generating hydroxyl radicals by single and two-frequency ultrasound with gold nanoparticles and protoporphyrin IX. Australas. Phys. Eng. Sci. Med. 2019, 42, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Noda, K.; Ogino, C.; Kuroda, S.; Shimizu, N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: Its application to targeted sonodynamic therapy. Ultrason. Sonochem. 2014, 21, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, K.; Lu, B.; Minakata, D.; Seo, Y.; Göktaş, R.K.; Dionysiou, D.D.; Tang, C.-J.; Wei, Z.; Spinney, R. Inactivation of pathogenic microorganisms by sulfate radical: Present and future. Chem. Eng. J. 2019, 371, 222–232. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A review study on sulfate-radical-based advanced oxidation processes for domestic/industrial wastewater treatment: Degradation, efficiency, and mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, A.K.-Y.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. A comprehensive review on persulfate activation treatment of wastewater. Sci. Tot. Environ. 2022, 831, 154906. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 2020, 54, 3064−3081. [Google Scholar] [CrossRef]
- Kermani, M.; Farzadkia, M.; Morovati, M.; Taghavi, M.; Fallahizadeh, S.; Khaksefidi, R.; Norzaee, S. Degradation of furfural in aqueous solution using activated persulfate and peroxymonosulfate by ultrasound irradiation. J. Environ. Manag. 2020, 266, 110616. [Google Scholar] [CrossRef] [PubMed]
- Venieri, D.; Karapa, A.; Panagiotopoulou, M.; Gounaki, I. Application of activated persulfate for the inactivation of fecal bacterial indicators in water. J. Environ. Manag. 2020, 261, 110223. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, G.; Zhu, J.-J. Sonochemical synthesis of Fe3O4/carbon nanotubes using low frequency ultrasonic devices and their performance for heterogeneous sono-persulfate process on inactivation of Microcystis aeruginosa. Ultrason. Sonochem. 2019, 58, 104634. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yan, L.; Xu, G.; Wang, X.; Wang, J.J.; Dionysios, D.D. High frequency ultrasonication enhances iron-catalyzed sulphate inactivation of Escherichia coli and Staphylococcus aureus. Chem. Eng. J. Adv. 2021, 8, 100170. [Google Scholar] [CrossRef]
- Tsenter, I.M.; Matafonova, G.G.; Batoev, V.B. Combination of high frequency ultrasound and UV radiation of excilamp for surface disinfection. Eng. Life Sci. 2015, 15, 830–834. [Google Scholar] [CrossRef]
- Tsenter, I.; Popova, S.; Garkusheva, N. Efficiency of sonophotolysis for eliminating bisphenol A and bacteria from aqueous solution. IOP Conf. Ser. Mater. Sci. Eng. 2020, 962, 042084. [Google Scholar] [CrossRef]
- Popova, S.; Tsenter, I.; Garkusheva, N.; Beck, S.; Matafonova, G.; Batoev, V. Evaluating (sono)-photo-Fenton-like processes with high-frequency ultrasound and UVA LEDs for degradation of organic micropollutants and inactivation of bacteria separately and simultaneously. J. Environ. Chem. Eng. 2021, 9, 105249. [Google Scholar] [CrossRef]
- Chang, J.D.; Wallace, A.G.; Foster, E.E.; Kim, S.J. Peptidoglycan compositional analysis of Enterococcus faecalis biofilm by stable isotope labeling by amino acids in bacterial culture. Biochemistry 2018, 57, 1274–1283. [Google Scholar] [CrossRef]
- Mason, T.J.; Cobley, A.J.; Graves, J.E.; Morgan, D. New evidence for the inverse dependence of mechanical and chemical effects on the frequency of ultrasound. Ultrason. Sonochem. 2011, 18, 226–230. [Google Scholar] [CrossRef]
- Hua, I.; Hoffmann, M.R. Optimization of ultrasonic irradiation as an advanced oxidation technology. Environ. Sci. Technol. 1997, 31, 2237–2243. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate constants and mechanism of reaction of sulfate radical anion with aromatic compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]
- Elovitz, M.S.; von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes. I. The Rct concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Eibenberger, H.; Steenken, S.; O’Neill, P.; Schulte-Frohlinde, D. Pulse radiolysis and electron spin resonance studies concerning the reaction of SO4·− with alcohols and ethers in aqueous solution. J. Phys. Chem. 1978, 82, 749−750. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrones, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Iacovou, M.; Frontistis, Z.; Hapeshi, E.; Dionysiou, D.D.; Fatta-Kassinos, D. Erythromycin oxidation and ERY-resistant Escherichia coli inactivation in urban wastewater by sulfate radical-based oxidation process under UV-C irradiation. Water Res. 2015, 85, 346–358. [Google Scholar] [CrossRef]
- Popova, S.; Matafonova, G.; Batoev, V. Simultaneous atrazine degradation and E. coli inactivation by UV/S2O82−/Fe2+ process under KrCl excilamp (222 nm) irradiation. Ecotoxicol. Environ. Saf. 2019, 169, 169–177. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, J.; Wang, Q.; Wang, D.; Xu, H.; Ma, J.; Qiu, W.; Hu, T. Sonolytic degradation of bisphenol S: Effect of dissolved oxygen and peroxydisulfate, oxidation products and acute toxicity. Water Res. 2019, 165, 114969. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, N.; Rawat, L.; Goyal, M.K. Effect of two waves of ultrasonic on waste water treatment. J. Chem. Eng. Process Technol. 2014, 5, 1000193. [Google Scholar]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T.W. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, K.; Cui, J.; Ye, J.Y.; Deng, C.X. Controlled permeation of cell membrane by single bubble acoustic cavitation. J. Control. Release 2012, 157, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.T.; Porter, T.M. Control of acoustic cavitation for efficient sonoporation with phase-shift nanoemulsions. Ultrasound Med. Biol. 2019, 45, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Shear stress in cells generated by ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 363–373. [Google Scholar] [CrossRef]
- Collis, J.; Manasseh, R.; Liovic, P.; Tho, P.; Ooi, A.; Petkovic-Duran, K.; Zhu, Y. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics 2010, 50, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical insight into mechanisms of sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef]
- Jia, C.; Xu, L.; Han, T.; Cai, P.; Yu, A.C.H.; Qin, P. Generation of reactive oxygen species in heterogeneously sonoporated cells by microbubbles with single-pulse ultrasound. Ultrasound Med. Biol. 2018, 44, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Tsenter, I.; Garkusheva, N.; Matafonova, G.; Batoev, V. A novel water disinfection method based on dual-wavelength UV radiation of KrCl (222 nm) and XeBr (282 nm) excilamps. J. Environ. Chem. Eng. 2022, 10, 107537. [Google Scholar] [CrossRef]
- Umemura, S.; Kawabata, K.; Sasaki, K. In vitro and in vivo enhancement of sonodynamically active cavitation by second-harmonic superimposition. J. Acoust. Soc. Am. 1997, 101, 569–577. [Google Scholar] [CrossRef]
- Jin, J.; Hayashi, T.; Miwa, T. Observation of noise generation induced in ultrasonic cavitation with ultramicroelectrode. Chem. Lett. 1998, 27, 539–540. [Google Scholar] [CrossRef]
- Petošić, A.; Ivančević, B.; Svilar, D. Measuring derived acoustic power of an ultrasound surgical device in the linear and nonlinear operating modes. Ultrasonics 2009, 49, 522–531. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, C. Single-transducer dual-frequency ultrasound generation to enhance acoustic cavitation. Ultrason. Sonochem. 2009, 16, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Li, S. Combination and simultaneous resonances of gas bubbles oscillating in liquids under dual-frequency acoustic excitation. Ultrason. Sonochem. 2017, 35, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhu, X.; Liu, Y. Numerical study on dual-frequency ultrasonic enhancing cavitation effect based on bubble dynamic evolution. Ultrason. Sonochem. 2019, 59, 104744. [Google Scholar] [CrossRef] [PubMed]





| Persulfate, mg/L | Sum of Log Reductions for Single-Frequency (Exposure Time) | φ |
|---|---|---|
| E. coli | ||
| 100 200 | 2.3 (60 min) 1.1 (30 min) | 2.3 5.0 |
| E. faecalis | ||
| 100 200 | 2.9 (100 min) 1.8 (60 min) | 1.8 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garkusheva, N.; Tsenter, I.; Kobunova, E.; Matafonova, G.; Batoev, V. Dual-Frequency Ultrasonic Inactivation of Escherichia coli and Enterococcus faecalis Using Persulfate: A Synergistic Effect. Water 2022, 14, 2604. https://doi.org/10.3390/w14172604
Garkusheva N, Tsenter I, Kobunova E, Matafonova G, Batoev V. Dual-Frequency Ultrasonic Inactivation of Escherichia coli and Enterococcus faecalis Using Persulfate: A Synergistic Effect. Water. 2022; 14(17):2604. https://doi.org/10.3390/w14172604
Chicago/Turabian StyleGarkusheva, Natalia, Irina Tsenter, Elena Kobunova, Galina Matafonova, and Valeriy Batoev. 2022. "Dual-Frequency Ultrasonic Inactivation of Escherichia coli and Enterococcus faecalis Using Persulfate: A Synergistic Effect" Water 14, no. 17: 2604. https://doi.org/10.3390/w14172604