Stored Reference Samples Enable Efficient Non-Target HRMS Screening for Novel Chemical Contamination in Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Samples and Work-Up
2.3. UHPLC-HRMS Analysis
2.4. GC-HRMS Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. RW Profile: Seasonal and Geographical Variation in the Profile of Organic Compounds
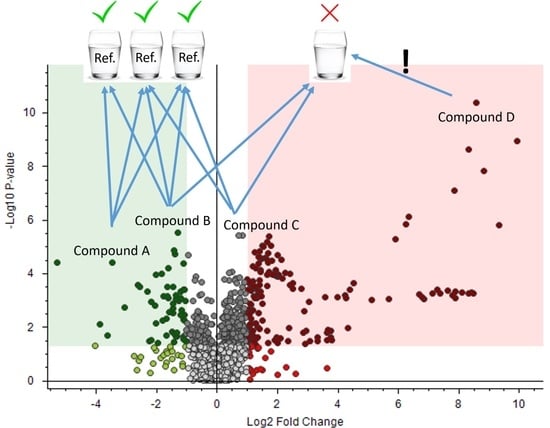
3.2. DW References: Comparison of Different Sets of Reference Samples for Drinking Water Analysis
3.3. 90 DWTPs: Influence of Raw Water and Processes of the DWTPs’ on the Profile of the Organic Compounds in the Drinking Water
3.4. Real Case: Application of the Suggested Methodology to a Real Case
3.5. Application of the Suggested Methodology to a Recent Exercise
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SWWA. The Swedish Water & Wastewater Association, Production of Drinking Water. 2019. Available online: http://www.svensktvatten.se/fakta-om-vatten/dricksvattenfakta/produktion-av-dricksvatten (accessed on 12 July 2022).
- Swedish Food Agency, Livsmedelsverket. Livsmedelsverkets Föreskrifter om Dricksvatten. 2001. Available online: https://www.livsmedelsverket.se/produktion-handel--kontroll/dricksvattenproduktion (accessed on 12 July 2022).
- Andersson, A.; Harir, M.; Gonsior, M.; Hertkorn, N.; Schmitt-Kopplin, P.; Kylin, H.; Karlsson, S.; Ashiq, M.J.; Lavonen, E.; Nilsson, K.; et al. Waterworks-specific composition of drinking water disinfection by-products. Environ. Sci. Water Res. Technol. 2019, 5, 861. [Google Scholar] [CrossRef] [Green Version]
- Schymanski, E.L.; Singer, H.P.; Slobodnik, J.; Ipolyi, I.M.; Oswald, P.; Krauss, M.; Schulze, T.; Haglund, P.; Letzel, T.; Grosse, S.; et al. Non-target screening with high-resolution mass spectrometry: Critical review using a collaborative trial on water analysis. Anal. Bioanal. Chem. 2015, 407, 6237–6255. [Google Scholar] [CrossRef] [PubMed]
- Letzel, T.; Bayer, A.; Schulz, W.; Heermann, A.; Lucke, T.; Greco, G.; Grosse, S.; Schüssler, W.; Sengl, M.; Letzel, M. LC–MS screening techniques for wastewater analysis and analytical data handling strategies: Sartans and their transformation products as an example. Chemosphere 2015, 137, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Castiglioni, S.; Covaci, A.; de Voogt, P.; Emke, E.; Kasprzyk-Hordern, B.; Ort, C.; Reid, M.; Sancho, J.V.; Thomas, V.K.; et al. Mass spectrometric strategies for the investigation of biomarkers of illicit drug use in wastewater. Mass Spectrom. Rev. 2018, 37, 258–280. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Samanipour, S.; Hollender, J.; Ibáñez, M.; Kaserzon, S.; Kokkali, V.; van Leerdam, J.; Mueller, J.F.; Pijnappels, M.; Reid, M.J.; et al. Exploring the Potential of a Global Emerging Contaminant Early Warning Network through the Use of Retrospective Suspect Screening with High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2018, 52, 5135–5144. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Rothardt, J.; Radny, D.; Loos, M.; Epting, J.; Huggenberger, P.; Borer, P.; Singer, H. Comprehensive micropollutant screening using LC-HRMS/MS at three riverbank filtration sites to assess natural attenuation and potential implications for human health. Water Res. X 2018, 1, 00007. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Oswald, P.; Thomaidis, N.S.; Schymanski, E.L.; Aalizadeh, R.; Schulze, T.; Oswaldova, M.; Slobodnik, J. NORMAN digital sample freezing platform: A European virtual platform to exchange liquid chromatography high resolution-mass spectrometry data and screen suspects in “digitally frozen” environmental samples. Trends Anal. Chem. 2019, 115, 129–137. [Google Scholar] [CrossRef]
- Hernandez, F.; Bakker, J.; Bijlsma, L.; de Boer, J.; Botero-Coy, A.M.; de Bruin, Y.B.; Fischer, S.; Hollender, J.; Kasprzyk-Hordern, B.; Lamoree, M.; et al. The role of analytical chemistry in exposure science: Focus on the aquatic environment. Chemosphere 2019, 222, 564–583. [Google Scholar] [CrossRef]
- Zahn, D.; Frömel, T. Finding a needle in a haystack—Analyte-driven tools and techniques for information extraction and prioritization of chemicals from environmental (chromatography-)HRMS nontarget screening data. Curr. Opin. Environ. Sci. Health 2020, 18, 70–78. [Google Scholar] [CrossRef]
- Herrmann, A.; Rosén, J.; Jansson, D.; Hellenäs, K.E. Evaluation of a generic multi-analyte method for detection of >100 representative compounds correlated to emergency events in 19 food types by ultrahigh-pressure liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1235, 115–124. [Google Scholar] [CrossRef]
- Mol, H.G.J.; Plaza-Bolaños, P.; Zomer, P.; De Rijk, T.C.; Stolker, A.A.M.; Mulder, P.P.J. Toward a generic extraction method for simultaneous determination of pesticides, mycotoxins, plant toxins, and veterinary drugs in feed and food matrixes. Anal. Chem. 2008, 80, 9450–9459. [Google Scholar] [CrossRef] [PubMed]
- ChemSpider. Available online: https://www.chemspider.com/ (accessed on 12 July 2022).
- Kunzelmann, M.; Winter, M.; Åberg, M.; Karl-Erik Hellenäs, K.-E.; Rosén, J. Non-targeted analysis of unexpected food contaminants using LC-HRMS. Anal. Bioanal. Chem. 2018, 410, 5593–5602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tengstrand, E.; Rosén, J.; Hellenäs, K.E.; Åberg, K.M. A concept study on non-targeted screening for chemical contaminants in food using liquid chromatography-mass spectrometry in combination with a metabolomics approach. Anal. Bioanal. Chem. 2013, 405, 1237–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, M.; Mueller, M.S.; Loos, M.; Singer, H.P. Quantitative target and systematic non-target analysis of polar organic micro-pollutants along the river Rhine using high-resolution massspectrometry e Identification of unknown sources and compounds. Water Res. 2015, 87, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Schymanski, E.L.; Singer, H.P.; Ferguson, P.L. Nontarget screening with high resolution mass spectrometry in the environment: Ready to go? Environ. Sci. Technol. 2017, 51, 11505–11512. [Google Scholar] [CrossRef] [Green Version]
- Bader, T.; Schulz, W.; Lucke, T.; Seitz, W.; Winzenbacher, R. Application of non-target analysis with LC-HRMS for the monitoring of raw and potable water: Strategy and results. In Assessing Transformation Products of Chemicals by Non-Target and Suspect Screening—Strategies and Workflows; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; Volume 1242, pp. 49–70. [Google Scholar] [CrossRef]
- Bader, T.; Schulz, W.; Kümmerer, K.; Winzenbacher, R. General strategies to increase the repeatability in non-target screening by liquid chromatography-high resolution mass spectrometry. Anal. Chim. Acta 2016, 935, 173–186. [Google Scholar] [CrossRef]
- Cui, X.; Churchill, G.A. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 2003, 4, 210. [Google Scholar] [CrossRef] [Green Version]
- Hur, M.; Campbell, A.A.; Almeida-de-Macedo, M.; Li, L.; Ransom, N.; Jose, A.; Crispin, M.; Nikolau, B.J.; Wurtele, E.S. A global approach to analysis and interpretation of metabolic data for plant natural product discovery. Nat. Prod. Rep. 2013, 30, 565–583. [Google Scholar] [CrossRef] [Green Version]
- EC. Drinking Water Legislation—Environment—European Commission. 2021. Available online: https://ec.europa.eu/environment/water/water-drink/legislation_en.html (accessed on 12 July 2022).
- Karki, A.J.; Cappelli, P.; Dirks, C.; Pekar, H.; Hellenäs, K.-E.; Rosén, J.; Westerberg, E. New efficient methodology for screening of selected organic micropollutants in raw- and drinking water from 90 Swedish water treatment plants. Sci. Total Environ. 2020, 724, 138069. [Google Scholar] [CrossRef]
- Tröger, R.; Köhler, S.J.; Franke, V.; Bergstedt, O.; Wiberg, K. A case study of organic micropollutants in a major Swedish water source—Removal efficiency in seven drinking water treatment plants and influence of operational age of granulated active carbon filters. Sci. Total Environ. 2020, 706, 135680. [Google Scholar] [CrossRef]
- Pfeifer, T.; Klaus, U.; Hoffmann, R.; Spiteller, M. Characterisation of humic substances using atmospheric pressure chemical ionisation and electrospray ionisation mass spectrometry combined with size-exclusion chromatography. J. Chromatogr. A 2001, 926, 151–159. [Google Scholar] [CrossRef]
- SWWA.2016. The Swedish Water & Wastewater Association and Norconsult AB, Gothenburg, Sweden. In Effektivare Fällning Vintertid vid Vattenverk med Höga Humushalter i Råvattnet; SWWA: Stockholm, Sweden, 2013; Available online: http://vav.griffel.net/filer/SVU-rapport_C_29-127.pdf (accessed on 12 July 2022).
- PubChem. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 12 July 2022).
- Norman Network. 2021. Available online: https://www.norman-network.net (accessed on 12 July 2022).







| Sample | Number of “False Positives” When Using Moderate Criteria (Fold Change > 8 and p-Value < 0.001) and 4 Months of Reference Water from: | Number of “False Positives” When Using Strict Criteria (Fold Change > 64 and p-Value < 1 × 10−5) and 4 Months of Reference Water from: | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DWTP | Month | Jönköping | Karlskrona | Karlstad | MQ | Jönköping | Karlskrona | Karlstad | MQ |
| Jönköping | Jan | 6 | 107 | 44 | 994 | 3 | 5 | 6 | 592 |
| Feb | 9 | 70 | 44 | 1004 | 2 | 2 | 5 | 571 | |
| Mar | 0 | 112 | 24 | 1004 | 0 | 4 | 6 | 586 | |
| Apr | 3 | 78 | 47 | 976 | 0 | 1 | 2 | 528 | |
| May | 11 | 127 | 47 | 1041 | 3 | 6 | 10 | 614 | |
| June | 13 | 86 | 60 | 927 | 0 | 5 | 5 | 451 | |
| July | 18 | 106 | 54 | 1029 | 9 | 5 | 9 | 580 | |
| Aug | 4 | 49 | 28 | 981 | 0 | 2 | 3 | 568 | |
| Sep | 12 | 118 | 52 | 1040 | 2 | 7 | 9 | 630 | |
| Oct | 1 | 51 | 30 | 1000 | 0 | 4 | 6 | 596 | |
| Nov | 3 | 120 | 27 | 1010 | 0 | 5 | 4 | 597 | |
| Dec | 2 | 45 | 35 | 977 | 0 | 3 | 4 | 550 | |
| Median | 5 | 96 | 44 | 1002 | 0 | 5 | 6 | 583 | |
| Karlskrona | Jan | 157 | 38 | 164 | 1035 | 25 | 23 | 32 | 619 |
| Mar | 125 | 7 | 120 | 1015 | 1 | 0 | 2 | 621 | |
| Apr | 134 | 0 | 86 | 983 | 3 | 0 | 1 | 611 | |
| May | 98 | 8 | 93 | 1015 | 0 | 0 | 1 | 648 | |
| June | 158 | 3 | 95 | 990 | 4 | 0 | 4 | 576 | |
| July | 126 | 5 | 121 | 1010 | 0 | 0 | 6 | 611 | |
| Sep | 62 | 10 | 45 | 999 | 0 | 0 | 0 | 616 | |
| Oct | 174 | 11 | 111 | 933 | 12 | 0 | 7 | 469 | |
| Nov | 186 | 28 | 169 | 1060 | 21 | 13 | 22 | 627 | |
| Dec | 127 | 0 | 70 | 957 | 5 | 0 | 0 | 580 | |
| Median | 131 | 8 | 103 | 1005 | 4 | 0 | 3 | 614 | |
| Karlstad | May | 38 | 15 | 9 | 1010 | 0 | 1 | 1 | 570 |
| June | 10 | 26 | 14 | 948 | 0 | 0 | 0 | 472 | |
| July | 45 | 12 | 10 | 977 | 0 | 1 | 0 | 536 | |
| Aug | 23 | 4 | 0 | 906 | 0 | 1 | 0 | 509 | |
| Sep | 35 | 16 | 1 | 982 | 0 | 1 | 0 | 554 | |
| Oct | 13 | 18 | 12 | 969 | 4 | 5 | 4 | 479 | |
| Nov | 22 | 28 | 25 | 980 | 6 | 1 | 4 | 508 | |
| Dec | 17 | 9 | 3 | 955 | 0 | 0 | 0 | 497 | |
| Median | 23 | 16 | 10 | 973 | 0 | 1 | 0 | 509 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosén, J.; Westerberg, E.; Pekar, H.; Cappelli, P.; Karki, A.J.; Mörén, L.; Åstot, C.; Hellenäs, K.-E. Stored Reference Samples Enable Efficient Non-Target HRMS Screening for Novel Chemical Contamination in Drinking Water. Water 2022, 14, 2586. https://doi.org/10.3390/w14162586
Rosén J, Westerberg E, Pekar H, Cappelli P, Karki AJ, Mörén L, Åstot C, Hellenäs K-E. Stored Reference Samples Enable Efficient Non-Target HRMS Screening for Novel Chemical Contamination in Drinking Water. Water. 2022; 14(16):2586. https://doi.org/10.3390/w14162586
Chicago/Turabian StyleRosén, Johan, Erik Westerberg, Heidi Pekar, Paolo Cappelli, Ajit Jung Karki, Lina Mörén, Crister Åstot, and Karl-Erik Hellenäs. 2022. "Stored Reference Samples Enable Efficient Non-Target HRMS Screening for Novel Chemical Contamination in Drinking Water" Water 14, no. 16: 2586. https://doi.org/10.3390/w14162586