Round-the-World Voyage of the Threespine Stickleback (Gasterosteus aculeatus): Phylogeographic Data Covering the Entire Species Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Isolation
2.3. Selection of Genetic Loci for Analysis
2.4. Polymerase Chain Reaction (PCR) and Sequencing of PCR Products
2.5. Analysis of the Sequenced COI and cyt b Fragments
2.6. COI and cyt b Sequences Deposited in the GenBank by Other Researchers
2.7. Construction of Median Networks of Haplotypes
3. Results
3.1. Median Networks of Haplotypes and Haplotype Distribution over the Species Range
3.2. Comparison of the Haplotype Networks for the Partial COI and cyt b Sequences
3.3. Distribution of Representatives of Different COI and cyt b Phylogenetic Lineages over the Species Range
3.4. Reconstruction of the Routes of Threespine Stickleback Dispersal within Its Range
3.5. Haplotypes found in Nontypical Localities
4. Discussion
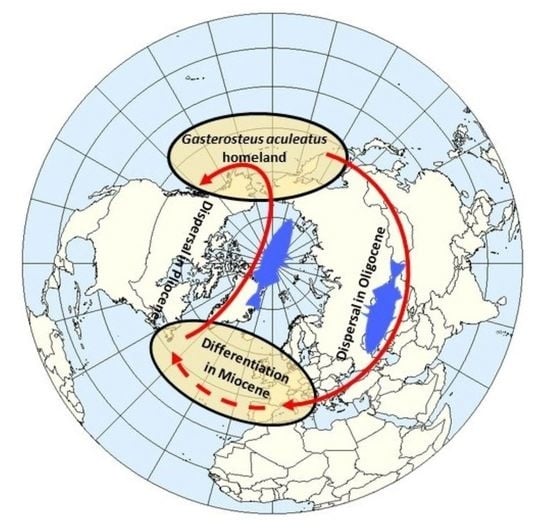
4.1. Direction of Threespine Stickleback Dispersal in the Course of the Formation of Its Modern Range and Irregularity in the Rates of Evolution
4.2. Time and Routes of the Invasion of the Threespine Stickleback into Europe
4.3. Differentiation of the Main Phylogenetic Lineages of Threespine Stickleback in the Atlantic Basin and the Spread of the Euro-North American Clade throughout the Range
4.4. Patterns of Geographic Location of the Haplotypes of the Transatlantic CTA/TA Lineage and the European CE/E Cluster Marking the Routes of Spread of G. aculeatus
4.5. Invasion of Carriers of the American Lineage Haplotypes into the Pacific Basin
4.6. Secondary Contacts between Representatives of Different Phylogenetic Lineages
4.7. Notes on the Origin of Gasterosteus wheatlandi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergström, U.; Olsson, J.; Casini, M.; Eriksson, B.K.; Fredriksson, R.; Wennhage, H.; Appelberg, M. Stickleback increase in the Baltic Sea—A thorny issue for coastal predatory fish. Estuar. Coast Shelf Sci. 2015, 163, 134–142. [Google Scholar] [CrossRef]
- Yurtseva, A.; Lüskow, F.; Hatton, M.; Doucet, A.; Lajus, D. Finfish vs jellyfish: Complimentary feeding patterns allow threespine stickleback Gasterosteus aculeatus and common jellyfish Aurelia aurita to co-exist in a Danish cove. Mar. Biol. 2018, 165, 148. [Google Scholar] [CrossRef]
- Lajus, D.L.; Golovin, P.V.; Zelenskaia, A.E.; Demchuk, A.S.; Dorgham, A.S.; Ivanov, M.V.; Ivanova, T.S.; Murzina, S.A.; Polyakova, N.V.; Rybkina, E.V.; et al. Threespine Stickleback of the White Sea: Population Characteristics and Role in the Ecosystem. Contemp. Probl. Ecol. 2020, 13, 132–145. [Google Scholar] [CrossRef]
- Hudson, C.M.; Lucek, K.; Marques, D.A.; Alexander, T.J.; Moosmann, M.; Spaak, P.; Seehausen, O.; Matthews, B. Threespine Stickleback in Lake Constance: The Ecology and Genomic Substrate of a Recent Invasion. Front. Ecol. Evol. 2021, 8, 611672. [Google Scholar] [CrossRef]
- Bell, M.A.; Foster, S.A. (Eds.) The Evolutionary Biology of the Threespine Stickleback; Oxford University Press: New York, NY, USA; Tokyo, Japan, 1994; pp. 1–571. [Google Scholar]
- Gibson, G. The synthesis and evolution of a supermodel. Science 2005, 307, 1890–1891. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.P.; Bolnick, D.I.; Berner, D.; Peichel, C.L. Along the speciation continuum in sticklebacks. J. Fish Biol. 2009, 75, 2000–2036. [Google Scholar] [CrossRef]
- Ivanova, T.S.; Ivanov, M.V.; Bakhvalova, A.E.; Polyakova, N.V.; Golovin, P.V.; Kucheryavyy, A.V.; Yurtseva, A.O.; Smirnova, K.A.; Lajus, D.L. Homing ability and site fidelity of marine threespine stickleback on spawning grounds. Evol. Ecol. Res. 2019, 20, 297–315. [Google Scholar]
- Terekhanova, N.V.; Barmintseva, A.E.; Kondrashov, A.S.; Bazykin, G.A.; Mugue, N.S. Architecture of Parallel Adaptation in Ten Lacustrine Threespine Stickleback Populations from theWhite Sea Area. Genome Biol. Evol. 2019, 11, 2605–2618. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Kemppainen, P.; Momigliano, P.; Feng, X.; Merilä, J. On the causes of geographically heterogeneous parallel evolution in sticklebacks. Nat. Ecol. Evol. 2020, 4, 1105–1115. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kitano, J. Diversity in reproductive seasonality in the three-spined stickleback, Gasterosteus aculeatus. J. Exp. Biol. 2020, 223, jeb208975. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.S.; Neretina, T.V.; Smirnova, O.V. Dynamics of Prolactin Axis Genes in the Brain of Male and Female Three-spined Stickleback Gasterosteus aculeatus (Gasterostaidae) during Short-Term Freshwater Adaptation. J. Ichthyol. 2020, 60, 299–304. [Google Scholar] [CrossRef]
- Artamonova, V.S.; Bardukov, N.V.; Golovin, P.V.; Ivanova, T.S.; Ivanov, M.V.; Lajus, D.L.; Makhrov, A.A. Determination of the female-biased sex ratio in some young-of-the-year and spawner samples of the threespine stickleback Gasterosteus aculeatus by environmental, not genetic, factors. Biol. Bull. 2021, 48, 577–587. [Google Scholar] [CrossRef]
- Ziuganov, V.V. The Family Gasterosteidae of World Fish Fauna; Nauka: Leningrad, Russia, 1991; pp. 1–261, (In Russian, summary in English). [Google Scholar]
- Higuchi, M.; Sakai, H.; Goto, A. A new threespine stickleback, Gasterosteus nipponicus sp. nov. (Teleostei: Gasterosteidae), from Japan Sea region. Ichthyol. Res. 2014, 61, 341–351. [Google Scholar] [CrossRef]
- Nazarkin, M.V.; Yabumoto, Y.; Urabe, A. A new Miocene three-spined stickleback (Pisces: Gasterosteidae) from Central Japan. Paleontol. Res. 2013, 16, 318–328. [Google Scholar] [CrossRef]
- Bennike, O. Quaternary vertebrates from Greenland: A review. Quat. Sci. Rev. 1997, 16, 899–909. [Google Scholar] [CrossRef]
- Haglund, T.R.; Buth, D.G.; Lawson, R. Allozyme variation and phylogenetic relationships of Asian, North American, and European populations of the threespine stickleback, Gasterosteus aculeatus. Copeia 1992, 1992, 432–443. [Google Scholar] [CrossRef]
- Orti, G.; Bell, M.A.; Reimchen, T.E.; Meyer, A. Global survey of mitochondrial DNA sequences in the threespine stickleback: Evidence for recent migrations. Evolution 1994, 48, 608–622. [Google Scholar] [CrossRef]
- Fang, B.; Merilä, J.; Matschiner, M.; Momigliano, P. Estimating uncertainty in divergence times among three-spined stickleback clades using the multispecies coalescent. Mol. Phylogenet. Evol. 2020, 142, 106646. [Google Scholar] [CrossRef]
- Fang, B.; Merilä, J.; Ribeiro, F.; Alexandre, C.M.; Momigliano, P. Worldwide phylogeny of three-spined sticklebacks. Mol. Phylogenet. Evol. 2018, 127, 613–625. [Google Scholar] [CrossRef]
- Heincke, F. Untersuchungen über die Stichlinge. Öfversigt at Kongl. Vetenskaps-Akademiens Förhandlingar. Stockholm. Arg. 1889, 46, 395–410. [Google Scholar]
- Huitfeldt-Kaas, H. Ferskvandsfiskenes Utbredelse og Invandring i Norge; Centraltrykkeriet: Kristiania, Norway, 1918; pp. 1–106. [Google Scholar]
- Thienemann, A. Die Süsswasserfische Deutschlands. Eine tiergeographische Skizze. In Handbuch der Binnenfisherie Mitteleuropas. Band III. Lieferung; Demoll, R., Maier, H.N., Eds.; G.m.b.H.: Stuttgart, Germany, 1926; pp. 1–32. [Google Scholar]
- Münzing, J. The evolution of variation and distributional patterns in European populations of the three-spined stickleback, Gasterosteus aculeatus. Evolution 1963, 17, 321–332. [Google Scholar] [CrossRef]
- Kosswig, C. Tethys and its relation to the peri-Mediterranean faunas of freshwater fishes. In Aspects of Tethyan Biogeography: A Symposium; Adams, C.G., Ager, D.V., Eds.; The Systematics Association: London, UK, 1967; pp. 313–324. [Google Scholar]
- Mäkinen, H.S.; Merilä, J. Mitochondrial DNA phylogeography of the three-spined stickleback (Gasterosteus aculeatus) in Europe—Evidence for multiple glacial refugia. Mol. Phylogenet. Evol. 2008, 46, 167–182. [Google Scholar] [CrossRef]
- DeFaveri, J.; Zanella, L.N.; Zanella, D.; Mrakovčić, M.; Merilä, J. Phylogeography of isolated freshwater three-spined stickleback Gasterosteus aculeatus populations in the Adriatic Sea basin. J. Fish Biol. 2012, 80, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Lucek, K.; Seehausen, O. Distinctive insular forms of threespine stickleback (Gasterosteus aculeatus) from western Mediterranean islands. Conserv. Genet. 2015, 16, 1319–1333. [Google Scholar] [CrossRef]
- Sanz, N.; Araguas, R.M.; Vidal, O.; Viñas, J. Glacial refuges for three-spined stickleback in the Iberian Peninsula: Mitochondrial DNA phylogeography. Freshw. Biol. 2015, 60, 1794–1809. [Google Scholar] [CrossRef]
- Vila, M.; Hermida, M.; Fernândez, C.; Perea, S.; Doadrio, I.; Amaro, R.; San Miguel, E. Phylogeography and conservation genetics of the Ibero-Balearic three-Spined stickleback (Gasterosteus aculeatus). PLoS ONE 2017, 12, e0170685. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Phil. Trans. R. Soc. B 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Artamonova, V.S.; Kolmakova, O.V.; Kirillova, E.A.; Makhrov, A.A. Phylogeny of salmonoid fishes (Salmonoidei) based on mtDNA COI gene sequences (barcoding). Contemp. Probl. Ecol. 2018, 11, 271–285. [Google Scholar] [CrossRef]
- Brown, T.A. Genomes 3, 3rd ed.; Garland Science: New York, NY, USA; London, UK, 2007; pp. 1–713. [Google Scholar]
- Geiger, M.F.; Herder, F.; Monaghan, M.T.; Almada, V.; Barbieri, R.; Bariche, M.; Berrebi, P.; Bohlen, J.; Casal-Lopez, M.; Delmastro, G.B.; et al. Spatial heterogeneity in the Mediterranean Biodiversity Hotspot affects barcoding accuracy of its freshwater fishes. Mol. Ecol. Resour. 2014, 14, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Kazmin, V.G.; Natapov, L.M. (Eds.) The Paleogeographic Atlas of Northern Eurasia; Institute of Tectonics of Lithospheric Plates: Moscow, Russia, 1998; 26 maps. [Google Scholar]
- Artamonova, V.S.; Bolotov, I.N.; Vinarski, M.V.; Makhrov, A.A. Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic. Water 2021, 13, 1161. [Google Scholar] [CrossRef]
- O’Reilly, P.; Reimchen, T.E.; Beech, R.; Strobeck, C. Mitochondrial DNA in Gasterosteus and Pleistocene Glacial Refugium on the Queen Charlotte Islands, British Columbia. Evolution 1993, 47, 678–684. [Google Scholar] [CrossRef]
- Deagle, B.E.; Reimchen, T.E.; Levin, D.B. Origins of endemic stickleback from the Queen Charlotte Islands: Mitochondrial and morphological evidence. Can. J. Zool. 1996, 74, 1045–1056. [Google Scholar] [CrossRef]
- Thompson, C.E.; Taylor, E.B.; McPhail, J.D. Parallel evolution of lake-stream pairs of threespine sticklebacks (Gasterosteus) inferred from mitochondrial DNA variation. Evolution 1997, 51, 1955–1965. [Google Scholar]
- Johnson, L.S.; Taylor, E.B. The distribution of divergent mitochondrial DNA lineages of threespine stickleback (Gasterosteus aculeatus) in the northeastern Pacific Basin: Post-glacial dispersal and lake accessibility. J. Biogeogr. 2004, 31, 1073–1083. [Google Scholar] [CrossRef]
- Lescak, E.A.; Marcotte, R.W.; Kenney, L.A.; Von Hippel, F.A.; Cresko, W.A.; Sherbick, M.L.; Colgren, J.J.; López, J.A. Admixture of ancient mitochondrial lineages in three-spined stickleback populations from the North Pacific. J. Biogeogr. 2015, 42, 532–539. [Google Scholar] [CrossRef]
- Gibbard, P.L. The history of the great northwest European rivers during the past three million years. Phil. Trans. R. Soc. Lond. B 1988, 318, 559–602. [Google Scholar]
- Torsvik, T.H.; Carlos, D.; Mosar, J.; Cocks, L.R.M.; Malme, T. Global reconstructions and North Atlantic palaeogeography 400 Ma to Recent. In BATLAS—Mid Norway Plate Reconstructions Atlas with Global and Atlantic Perspectives; Eide, E.A., Ed.; Geological Survey of Norway: Trondheim, Norway, 2002; pp. 18–39. [Google Scholar]
- Popov, S.V.; Rögl, F.; Rozanov, A.Y.; Steininger, F.F.; Shcherba, I.G.; Kovac, M. (Eds.) Lithological-Paleogeographic Maps of Paratethys: 10 Maps Late Eocene to Pliocene; CFS: Victoria, BC, Canada, 2004; Volume 250, pp. 1–46. [Google Scholar]
- Sanz, N. Phylogeographic history of brown trout: A review. In Brown Trout: Biology, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 17–63. [Google Scholar]
- Makhrov, A.A.; Bolotov, I.N. Ecological causes of high morphological plasticity of members of a taxon inhabiting the center of its origin (Exemplified by the Noble Salmons, genus Salmo). Biol. Bull. 2019, 46, 38–46. [Google Scholar] [CrossRef]
- Artamonova, V.S.; Afanasyev, S.A.; Bardukov, N.V.; Golod, V.M.; Kokodiy, S.V.; Koulish, A.V.; Pashkov, A.N.; Pipoyan, S.K.; Reshetnikov, S.I.; Makhrov, A.A. The Center of Origin and Colonization Routes of Noble Salmons of the Genus Salmo (Salmonidae, Actinopterigii). Dokl. Biochem. Biophys. 2020, 493, 171–177. [Google Scholar] [CrossRef]
- Gibson, D.I.; Køie, M. Magnibursatus caudofilamentosa (Reimer, 1971) n. comb. (Digenea: Derogenidae) from the stickleback Gasterosteus aculeatus L. in Danish waters: A zoogeographical anomaly? Syst. Parasitol. 1991, 20, 221–228. [Google Scholar] [CrossRef]
- Zander, C.D. Parasite diversity of sticklebacks from the Baltic Sea. Parasitol. Res. 2007, 100, 287–297. [Google Scholar] [CrossRef]
- Kostadinova, A.; Power, A.M.; Fernández, M.; Balbuena, J.A.; Raga, J.A.; Gibson, D.I. Three species of Magnibursatus Naidenova, 1969 (Digenea: Derogenidae) from Atlantic and Black Sea marine teleosts. Folia Parasitol. 2003, 50, 202–210. [Google Scholar] [CrossRef]
- Kostadinova, A.; Gibson, D.I. New records of rare derogenids (Digenea: Hemiuroidea) from Mediterranean sparids, including the description of a new species of Magnibursatus Naidenova, 1969 and redescription of Derogenes adriaticus Nikolaeva. Syst. Parasitol. 2009, 74, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.A.; Lucek, K.; Sousa, V.C.; Excoffier, L.; Seehausen, O. Admixture between old lineages facilitated contemporary ecological speciation in Lake Constance stickleback. Nat. Commun. 2019, 10, 4240. [Google Scholar] [CrossRef] [PubMed]
- Fredskild, B.; Røen, U. Macrofossils in an interglacial peat deposit at Kap Kfibenhavn, North Greenland. Boreas 1982, 11, 181–185. [Google Scholar] [CrossRef]
- Taylor, E.B.; McPhail, J.D. Evolutionary history of an adaptive radiation in species pairs of threespine sticklebacks (Gasterosteus): Insights from mitochondrial DNA. Biol. J. Linnaean Soc. 1999, 66, 271–291. [Google Scholar] [CrossRef]
- Watanabe, K.; Mori, S.; Nishida, M. Genetic relationships and origin of two geographic groups of freshwater threespine sticklebacks, ‘Hariyo’. Zool. Sci. 2003, 20, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Lescak, E.A.; Wund, M.A.; Bassham, S.; Catchen, J.; Prince, D.J.; Lucas, R.; Dominguez, G.; Von Hippel, F.A.; Cresko, W.A. Ancient three-spined stickleback (Gasterosteus aculeatus) mtDNA lineages are not associated with phenotypic or nuclear genetic variation. Biol. J. Linn. Soc. 2017, 122, 579–588. [Google Scholar] [CrossRef]
- Rezansoff, A.M.; Crispo, E.; Blair, C.; Cruz, E.; Kitano, J.; Vamosi, S.M.; Rogers, S.M. Toward the genetic origins of a potentially non-native population of threespine stickleback (Gasterosteus aculeatus) in Alberta. Conserv. Genet. 2015, 16, 859–873. [Google Scholar] [CrossRef]
- Richmond, J.Q.; Jacobs, D.K.; Backlin, A.R.; Swift, C.C.; Dellith, C.; Fisher, R.N. Ephemeral stream reaches preserve the evolutionary and distributional history of threespine stickleback in the Santa Clara and Ventura River watersheds of southern California. Conserv. Genet. 2015, 16, 85–101. [Google Scholar] [CrossRef]
- Strobel, H.M.; Alda, F.; Sprehn, C.G.; Blum, M.J.; Heins, D.C. Geographic and host-mediated population genetic structure in a cestode parasite of the three-spined stickleback. Biol. J. Linn. Soc. 2016, 119, 381–396. [Google Scholar] [CrossRef]
- Rahaman, W.; Smik, L.; Köseoğlu, D.N.L.; Tarique, M.; Thamban, M.; Haywood, A.; Belt, S.T.; Knies, J. Reduced Arctic sea ice extent during the mid-Pliocene Warm Period concurrent with increased Atlantic-climate regime. Earth Planet. Sci. Lett. 2020, 550, 116535. [Google Scholar] [CrossRef]
- Lumme, J.; Mäkinen, H.; Ermolenko, A.V.; Gregg, J.L.; Ziętara, M.S. Displaced phylogeographic signals from Gyrodactylus arcuatus, a parasite of the three-spined stickleback Gasterosteus aculeatus, suggest freshwater glacial refugia in Europe. Int. J. Parasitol. 2016, 46, 545–554. [Google Scholar] [CrossRef]
- Vermeij, G.J.; Banker, R.; Capece, L.R.; Hernandez, E.S.; Salley, S.O.; Vriesman, V.P.; Wortham, B.E. The coastal North Pacific: Origins and history of a dominant marine biota. J. Biogeogr. 2019, 46, 1–18. [Google Scholar] [CrossRef]
- Laakkonen, H.M.; Hardman, M.; Strelkov, P.; Väinölä, R. Cycles of trans-Arctic dispersal and vicariance, and diversification of the amphi-boreal marine fauna. J. Evol. Biol. 2021, 34, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Koretsky, I.A.; Rahmat, S.J. First records of fossil Cystophorinae (Carnivora, Phocidae): Middle miocene seals from the Northern Paratethys. Riv. Ital. Paleontol. 2013, 119, 325–350. [Google Scholar]
- Ziegler, P.A.; Fraefel, M. Response of drainage systems to Neogene evolution of the Jura fold-thrust belt and Upper Rhine Graben. Swiss J. Geosci. 2009, 102, 57–75. [Google Scholar] [CrossRef]
- Winterberg, S.; Willett, S.D. Greater Alpine river network evolution, interpretations based on novel drainage analysis. Swiss J. Geosci. 2019, 112, 3–22. [Google Scholar] [CrossRef]
- Berg, L.S. Übersicht der Verbreitung der Süsswasserfische Europas. Zoogeographica 1932, 1, 107–208. [Google Scholar]
- Denys, G.P.J.; Persat, H.; Dettai, A.; Geiger, M.F.; Freyhof, J.; Fesquet, J.; Keith, P. Genetic and morphological discrimination of three species of ninespined stickleback Pungitius spp. (Teleostei, Gasterosteidae) in France with the revalidation of Pungitius vulgaris (Mauduyt, 1848). J. Zool. Syst. Evol. Res. 2018, 56, 77–101. [Google Scholar] [CrossRef]
- Persat, H.; Mattersdorfer, K.; Charlat, S.; Schenekar, T.; Weiss, S. Genetic integrity of the European grayling (Thymallus thymallus) populations within the Vienne River drainage basin after five decades of stockings. Cybium 2016, 40, 7–20. [Google Scholar]
- Makhrov, A.A.; Verspoor, E.; Artamonova, V.S.; O’Sullivan, M. Atlantic salmon colonization of the Russian Arctic coast: Pioneers from North America. J. Fish Biol. 2005, 67, 68–79. [Google Scholar] [CrossRef]
- Jones, D.H.; John, A.W.G. A three-spined stickleback, Gasterosteus aculeatus L. from the North Atlantic. J. Fish Biol. 1978, 13, 231–236. [Google Scholar] [CrossRef]
- Quinn, T.P.; Light, J.T. Occurrence of threespine sticklebacks (Gasterosteus aculeatus) in the open North Pacific Ocean: Migration or drift? Can. J. Zool. 1989, 67, 2850–2852. [Google Scholar] [CrossRef]
- Denys, G.P.J.; Geiger, M.F.; Persat, H.; Dettai, A. Invalidity of Gasterosteus gymnurus (Cuvier, 1829) (Actinopterygii, Gasterosteidae) according to integrative taxonomy. Cybium 2015, 39, 37–45. [Google Scholar]
- Adachi, T.; Ishikawa, A.; Mori, S.; Makino, W.; Kume, M.; Kawata, M.; Kitano, J. Shifts in morphology and diet of non-native sticklebacks introduced into Japanese crater lakes. Ecol. Evol. 2012, 2, 1083–1098. [Google Scholar] [CrossRef]
- Paepke, H.-J. Die Stichlinge: Gasterosteidae; Westarp-Wiss: Magdeburg, Germany, 1996; pp. 1–176. [Google Scholar]
- Mattern, M.Y. Phylogeny, systematics, and taxonomy of sticklebacks. In Biology of the Three-Spined Stickleback; Ostlund-Nilsson, S., Mayer, I., Huntingford, F.A., Eds.; CRC Press Taylor & Francis Group: Boca Raton, CA, USA, 2007; pp. 1–40. [Google Scholar]
- Nesis, K.N. Pacific elements in northwest Atlantic benthos. In Soviet Fisheries Investigations in the Northwest Atlantic; Marti, Y., Ed.; Israel Program for Scientific Translations: Jerusalem, Israel, 1963; pp. 82–99. [Google Scholar]
- Makhrov, A.A.; Lajus, D.L. Postglacial colonization of the North European seas by Pacific fishes and lamprey. Contemp. Probl. Ecol. 2018, 11, 247–258. [Google Scholar] [CrossRef]
- Sardell, J.M.; Josephson, M.P.; Dalziel, A.C.; Peichel, C.L.; Kirkpatrick, M. Heterogeneous histories of recombination suppression on stickleback sex chromosomes. Mol. Biol. Evol. 2021, 38, 4403–4418. [Google Scholar] [CrossRef]
- Roe, K.J.; Harris, P.M.; Mayden, R.L. Phylogenetic relationships of the genera of North American sunfishes and basses (Percoidei: Centrarchidae) as evidenced by the mitochondrial cytochrome b gene. Copeia 2002, 2002, 897–905. [Google Scholar] [CrossRef]
- Wilson, A.B.; Vincent, A.; Ahnesjo, I.; Meyer, A. Male pregnancy in seahorses and pipefishes (family Syngnathidae): Rapid diversification of paternal brood pouch morphology inferred from a molecular phylogeny. J. Hered. 2001, 92, 159–166. [Google Scholar] [CrossRef]
- Wang, C.; Shikano, T.; Persat, H.; Merilä, J. Mitochondrial phylogeography and cryptic divergence in the stickleback genus Pungitius. J. Biogeogr. 2015, 42, 2334–2348. [Google Scholar] [CrossRef]
- Lucek, K.; Roy, D.; Bezault, E.; Sivasundar, A.; Seehausen, O. Hybridization between distant lineages increases adaptive variation during a biological invasion: Stickleback in Switzerland. Mol. Ecol. 2010, 19, 3995–4011. [Google Scholar] [CrossRef]
- Takahashi, H.; Møller, P.R.; Shedko, S.V.; Ramatulla, T.; Joen, S.R.; Zhang, C.G.; Sideleva, V.G.; Takata, K.; Sakai, H.; Goto, A.; et al. Species phylogeny and diversification process of Northeast Asian Pungitius revealed by AFLP and mtDNA markers. Mol. Phylogenet. Evol. 2016, 99, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Hanner, R.; Holm, E.; Mandrak, N.E.; Taylor, E.; Burridge, M.; Watkinson, D.; Dumont, P.; Curry, A.; Bentzen, P.; et al. Identifying Canadian Freshwater Fishes through DNA Barcodes. PLoS ONE 2008, 3, E2490. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, C.W.; Møller, P.R.; Steinke, D. Biodiversity of arctic marine fishes: Taxonomy and zoogeography. Mar. Biodiv. 2011, 41, 109–140. [Google Scholar] [CrossRef]
- McCusker, M.R.; Denti, D.; Van Guelpen, L.; Kenchington, E.; Bentzen, P. Barcoding Atlantic Canada’s commonly encountered marine fishes. Mol. Ecol. Resour. 2013, 13, 177–188. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artamonova, V.S.; Bardukov, N.V.; Aksenova, O.V.; Ivanova, T.S.; Ivanov, M.V.; Kirillova, E.A.; Koulish, A.V.; Lajus, D.L.; Malyutina, A.M.; Pashkov, A.N.; et al. Round-the-World Voyage of the Threespine Stickleback (Gasterosteus aculeatus): Phylogeographic Data Covering the Entire Species Range. Water 2022, 14, 2484. https://doi.org/10.3390/w14162484
Artamonova VS, Bardukov NV, Aksenova OV, Ivanova TS, Ivanov MV, Kirillova EA, Koulish AV, Lajus DL, Malyutina AM, Pashkov AN, et al. Round-the-World Voyage of the Threespine Stickleback (Gasterosteus aculeatus): Phylogeographic Data Covering the Entire Species Range. Water. 2022; 14(16):2484. https://doi.org/10.3390/w14162484
Chicago/Turabian StyleArtamonova, Valentina S., Nikolay V. Bardukov, Olga V. Aksenova, Tatiana S. Ivanova, Mikhail V. Ivanov, Elizaveta A. Kirillova, Andrey V. Koulish, Dmitry L. Lajus, Anna M. Malyutina, Andrey N. Pashkov, and et al. 2022. "Round-the-World Voyage of the Threespine Stickleback (Gasterosteus aculeatus): Phylogeographic Data Covering the Entire Species Range" Water 14, no. 16: 2484. https://doi.org/10.3390/w14162484