An Online-SPE/SEC/LCMS Method for the Detection of N-Nitrosamine Disinfection Byproducts in Wastewater Plant Tailwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
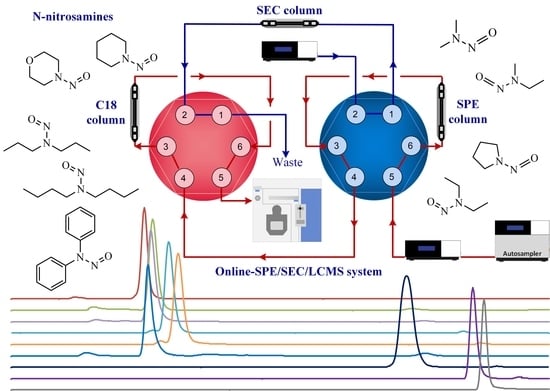
2.2. Setup of the Online-SPE/SEC/LCMS System
2.3. Chemical Reagents and Standard Solution Preparation
2.4. Standard Addition Method for Calibration
2.5. Chromatography Conditions Optimization
2.6. Mass Spectrometry Conditions Optimization
2.7. Method Validation
2.8. Usability of the Method
3. Results and Discussion
3.1. Selection of Chromatographic Column
3.2. Selection of Mobile Phase
3.3. Mass Spectrometry Parameter Optimization
3.4. Method Validation
3.5. Matrix Effect Evaluation
3.6. Method Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Ding, S.; Chu, W. Recent advances in the analysis of nitrogenous disinfection by-products. Trends Environ. Anal. Chem. 2017, 14, 19–27. [Google Scholar] [CrossRef]
- Piazzoli, A.; Breider, F.; Aquillon, C.G.; Antonelli, M.; von Gunten, U. Specific and total N-nitrosamines formation potentials of nitrogenous micropollutants during chloramination. Water Res. 2018, 135, 311–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Cheng, H. Degradation of N-nitrosodimethylamine (NDMA) and its precursor dimethylamine (DMA) in mineral micropores induced by microwave irradiation. Water Res. 2016, 94, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-H.; Mitch, W.A. Effect of Ozonation and Biological Activated Carbon Treatment of Wastewater Effluents on Formation of N-nitrosamines and Halogenated Disinfection Byproducts. Environ. Sci. Technol. 2017, 51, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, Y.; Hu, Y.; Hanigan, D.; Westerhoff, P.; An, D. Formation and control of C- and N-DBPs during disinfection of filter backwash and sedimentation sludge water in drinking water treatment. Water Res. 2021, 194, 116964. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, Y.; Zhang, X.; Yang, X. Treating disinfection byproducts with UV or solar irradiation and in UV advanced oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124435. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, T.; Zhang, J.; Li, Q.; Qin, Y.; Chen, L.; Zhang, Z. Occurrence and fate of N-nitrosamines in three full-scale drinking water treatment systems with different treatment trains. Sci. Total Environ. 2021, 783, 146982. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, L.; Huang, Y.; Spencer, P.; Zheng, W.; Zhou, Y.; Jiang, S.; Ye, W.; Zheng, Y.; Qu, W. Carcinogenic risk of N-Nitrosamines in Shanghai Drinking Water: Indications for the Use of Ozone Pretreatment. Environ. Sci. Technol. 2019, 53, 7007–7018. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Q.; Song, H.; Zhang, J.; Wang, L.; Qi, J.; Liu, Y.; Ma, J. Comparative study about oxidation of trace N-nitrosamines by seven oxidation processes with a sensitivity improved determination method. Sep. Purif. Technol. 2020, 236, 116009. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Gu, Y.; Pan, E.; Sun, Z.; Zhang, H.; Lu, Q.; Zhang, Y.; Yu, X.; Liu, R.; et al. Urinary exposure of N-nitrosamines and associated risk of esophageal cancer in a high incidence area in China. Sci. Total Environ. 2020, 738, 139713. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Huang, H.; Lu, Y.; Khorram, M.S.; Zhao, W.; Wang, D.; Qi, S.; Jin, B.; Zhang, G. Occurrence of N-Nitrosamines in the Pearl River delta of China: Characterization and evaluation of different sources. Water Res. 2019, 164, 114896. [Google Scholar] [CrossRef] [PubMed]
- Liew, D.; Linge, K.L.; Joll, C.A. Formation of nitrogenous disinfection by-products in 10 chlorinated and chloraminated drinking water supply systems. Environ. Monit. Assess. 2016, 188, 518. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y.; Basheer, C.; Lee, H.K. Ordered mesoporous carbon as sorbent for the extraction of N-nitrosamines in wastewater and swimming pool water. J. Chromatogr. A 2017, 1513, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Bei, E.; Wang, Y.; Wang, J.; Zhang, X.; Chen, C. One representative water supply system in China with nitrosamine concern: Challenges and treatment strategies. J. Environ. Sci. 2020, 88, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.J.; Insa, S.; Lamb, A.; Cojocariu, C.; Gernjak, W. Occurrence of N-nitrosamines and their precursors in Spanish drinking water treatment plants and distribution systems. Environ. Sci. Water Res. Technol. 2020, 6, 210–220. [Google Scholar] [CrossRef]
- Sgroi, M.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. N-Nitrosodimethylamine (NDMA) and its precursors in water and wastewater: A review on formation and removal. Chemosphere 2018, 191, 685–703. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Huang, H.; Zhu, X.; Jiang, X.; Zhang, Y.; Cen, K.; Zhao, L.; Liu, X.; Qi, S. Comparison of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry with electron ionization for determination of N-nitrosamines in environmental water. Chemosphere 2017, 168, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Wang, C.; Ying, Z.; Fan, W.; Yang, W. Occurrence and formation potential of nitrosamines in river water and ground water along the Songhua River, China. J. Environ. Sci. 2016, 50, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Amayreh, M. Determination of N-nitrosamines in Water by Automated Headspace Solid-Phase Microextraction. Arab. J. Sci. Eng. 2019, 44, 269–278. [Google Scholar] [CrossRef]
- Sgroi, M.; Roccaro, P.; Oelker, G.; Snyder, S.A. N-nitrosodimethylamine (NDMA) formation during ozonation of wastewater and water treatment polymers. Chemosphere 2016, 144, 1618–1623. [Google Scholar] [CrossRef]
- Gushgari, A.J.; Halden, R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 2018, 210, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Wang, Z.; Zhang, Q.; Bai, H.; Lv, Q. High resolution GC–Orbitrap MS for nitrosamines analysis: Method performance, exploration of solid phase extraction regularity, and screening of children’s products. Microchem. J. 2021, 162, 105878. [Google Scholar] [CrossRef]
- Kühne, F.; Kappenstein, O.; Straβgütl, S.; Weese, F.; Weyer, J.; Pfaff, K.; Luch, A. N-nitrosamines migrating from food contact materials into food simulants: Analysis and quantification by means of HPLC-APCI-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Chisvert, A.; Salvador, A. Determination of N-nitrosamines in cosmetic products by vortex-assisted reversed-phase dispersive liquid-liquid microextraction and liquid chromatography with mass spectrometry. J. Sep. Sci. 2018, 41, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Ngongang, A.D.; Duy, S.V.; Sauvé, S. Analysis of nine N-nitrosamines using liquid chromatography-accurate mass high resolution-mass spectrometry on a Q-Exactive instrument. Anal. Methods 2015, 7, 5748–5759. [Google Scholar] [CrossRef]
- Malihi, F.; Wang, T. An improved analytical method for quantitation of nitrosamine impurities in ophthalmic solutions using liquid chromatography with tandem mass spectrometry. J. Chromatogr. Open 2022, 2, 100037. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, D.; Wang, B.; Wang, Z.; Wang, Z. Rapid and sensitive method for simultaneous determination of nine N-nitrosamines in drinking water by UPLC-MS/MS. Sci. Sin.-Chim. 2011, 41, 82–90. [Google Scholar] [CrossRef]
- Sluggett, G.W.; Zelesky, T.; Hetrick, E.M.; Babayan, Y.; Baertschi, S.W. Artifactual degradation of secondary amine-containing drugs during accelerated stability testing when saturated sodium nitrite solutions are used for humidity control. J. Pharm. Biomed. Anal. 2018, 149, 206–213. [Google Scholar] [CrossRef]
- Alexandrou, L.D.; Bowen, C.; Jones, O.A.H. Fast analysis of multiple haloacetic acids and nitrosamines in recycled and environmental waters using liquid chromatography-mass spectrometry with positive–negative switching and multiple reaction monitoring. Anal. Methods 2019, 11, 3793–3799. [Google Scholar] [CrossRef]
- Kürzl, C.; Wohlschläger, H.; Schiffer, S.; Kulozik, U. Concentration, purification and quantification of milk protein residues following cleaning processes using a combination of SPE and RP-HPLC. MethodsX 2022, 9, 101695. [Google Scholar] [CrossRef]
- Rach, O.; Hadeen, X.; Sachse, D. An automated solid phase extraction procedure for lipid biomarker purification and stable isotope analysis. Org. Geochem. 2020, 142, 103995. [Google Scholar] [CrossRef]
- Qian, Y.; Wu, M.; Wang, W.; Chen, B.; Zheng, H.; Krasner, S.W.; Hrudey, S.E.; Li, X.-F. Determination of 14 nitrosamines at nanogram per liter levels in drinking water. Anal. Chem. 2015, 87, 1330–1336. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, J.; Zhong, Q.; Yu, D.; Yao, J.; Huang, T.; Zhu, M.; Zhou, T. A fully automatic cross used solid-phase extraction online coupled with ultra-high performance liquid chromatography-tandem mass spectrometry system for the trace analysis of multi-class pharmaceuticals in water samples. J. Pharm. Biomed. Anal. 2019, 174, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Hama, J.R.; Strobel, B.W. Pyrrolizidine alkaloids quantified in soil and water using UPLC-MS/MS. RSC Adv. 2019, 9, 30350–30357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciel, E.V.S.; de Toffoli, A.L.; Lanças, F.M. Current status and future trends on automated multidimensional separation techniques employing sorbent-based extraction columns. J. Sep. Sci. 2019, 42, 258–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusmaroli, L.; Insa, S.; Petrovic, M. Development of an online SPE-UHPLC-MS/MS method for the multiresidue analysis of the 17 compounds from the EU “Watch list”. Anal. Bioanal. Chem. 2018, 410, 4165–4176. [Google Scholar] [CrossRef]
- Marasco Júnior, C.A.; Sartore, D.M.; Lamarca, R.S.; Da Silva, B.F.; Santos-Neto, Á.J.; de Lima Gomes, P.C.F. On-line solid-phase extraction of pharmaceutical compounds from wastewater treatment plant samples using restricted access media in column-switching liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1180, 122896. [Google Scholar] [CrossRef]
- Amelin, V.G.; Bol’shakov, D.S. Rapid Identification and Determination of N-Nitrosamines in Food Products by Ultra-High-Performance Liquid Chromatography–High Resolution Quadrupole-Time-of-Flight Mass Spectrometry by Exact Masses of Protonated Molecules. J. Anal. Chem. 2019, 74, 39–46. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef]
- Dubuis, A.; Le Masle, A.; Chahen, L.; Destandau, E.; Charon, N. Off-line comprehensive size exclusion chromatography × reversed-phase liquid chromatography coupled to high resolution mass spectrometry for the analysis of lignocellulosic biomass products. J. Chromatogr. A 2020, 1609, 460505. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Beck, A.; Veuthey, J.-L.; Guillarme, D. Theory and practice of size exclusion chromatography for the analysis of protein aggregates. J. Pharm. Biomed. Anal. 2014, 101, 161–173. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Chi, L.-M.; Chien, K.-Y. Size-exclusion chromatography using reverse-phase columns for protein separation. J. Chromatogr. A 2018, 1571, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Park, J.D.; Kim, D.H.; Hartmann, R.W. A method for screening enzyme inhibitors using size exclusion chromatography and ESI-LC-MS/MS. J. Biomol. Screen. 2005, 10, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusotti, G.; Calleri, E.; Colombo, R.; Massolini, G.; Rinaldi, F.; Temporini, C. Advances on Size Exclusion Chromatography and Applications on the Analysis of Protein Biopharmaceuticals and Protein Aggregates: A Mini Review. Chromatographia 2018, 81, 3–23. [Google Scholar] [CrossRef]
- Saraswat, M.; Garapati, K.; Mun, D.-G.; Pandey, A. Extensive heterogeneity of glycopeptides in plasma revealed by deep glycoproteomic analysis using size-exclusion chromatography. Mol. Omics 2021, 17, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; Kim, M.; Dai, L.; Cornell, C.; Jacobson, F.; Guillarme, D.; Stella, C. Streamlined Characterization of an Antibody-Drug Conjugate by Two-Dimensional and Four-Dimensional Liquid Chromatography/Mass Spectrometry. Anal. Chem. 2019, 91, 14896–14903. [Google Scholar] [CrossRef]
- Chilakala, S.; Mehtab, V.; Tallapally, M.; Vemula, M.; Shaikh, A.S.; Chenna, S.; Upadhyayula, V. SEC-MS/MS determination of amino acids from mango fruits and application of the method for studying amino acid perturbations due to post harvest ripening. LWT 2021, 138, 110680. [Google Scholar] [CrossRef]
- Hewavitharana, A.K.; Abu Kassim, N.S.; Shaw, P.N. Standard addition with internal standardisation as an alternative to using stable isotope labelled internal standards to correct for matrix effects-Comparison and validation using liquid chromatography-tandem mass spectrometric assay of vitamin D. J. Chromatogr. A 2018, 1553, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sloop, J.T.; Gonçalves, D.A.; O’Brien, L.M.; Carter, J.A.; Jones, B.T.; Donati, G.L. Evaluation of different approaches to applying the standard additions calibration method. Anal. Bioanal. Chem. 2021, 413, 1293–1302. [Google Scholar] [CrossRef]
- Syed, H.K.; Liew, K.B.; Loh, G.O.K.; Peh, K.K. Stability indicating HPLC-UV method for detection of curcumin in Curcuma longa extract and emulsion formulation. Food Chem. 2015, 170, 321–326. [Google Scholar] [CrossRef]
- Patil, M.P.N. HPLC Method Development-A Review. J. Pharm. Res. Educ. 2017, 1, 243–260. [Google Scholar]
- Ćirić, A.; Prosen, H.; Jelikić-Stankov, M.; Đurđević, P. Evaluation of matrix effect in determination of some bioflavonoids in food samples by LC-MS/MS method. Talanta 2012, 99, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; White, R.L. Reducing glycerophosphocholine lipid matrix interference effects in biological fluid assays by using high-turbulence liquid chromatography. Rapid Commun. Mass Spectrom. 2008, 22, 3362–3370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, W.-Q.; Ding, Y.-Q.; Zhu, B.-Q.; Fan, Z.-Y.; Luo, J.-W. Rapid determination of sulfonamides in chicken using two-dimensional online cleanup mode with three columns coupled to liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1114–1115, 110–118. [Google Scholar] [CrossRef]
- Komatsu, K.; Onodera, T.; Kohzu, A.; Syutsubo, K.; Imai, A. Characterization of dissolved organic matter in wastewater during aerobic, anaerobic, and anoxic treatment processes by molecular size and fluorescence analyses. Water Res. 2020, 171, 115459. [Google Scholar] [CrossRef]
- McAdams, B.C.; Aiken, G.R.; McKnight, D.M.; Arnold, W.A.; Chin, Y.-P. High Pressure Size Exclusion Chromatography (HPSEC) Determination of Dissolved Organic Matter Molecular Weight Revisited: Accounting for Changes in Stationary Phases, Analytical Standards, and Isolation Methods. Environ. Sci. Technol. 2018, 52, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, L. Variations in Colloidal DOM Composition with Molecular Weight within Individual Water Samples as Characterized by Flow Field-Flow Fractionation and EEM-PARAFAC Analysis. Environ. Sci. Technol. 2020, 54, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Auclair, J.; Bhattacharya, S.; Sarin, D. Two-Dimensional Liquid Chromatography (2D-LC): Analysis of Size-Based Heterogeneities in Monoclonal Antibody–Based Biotherapeutic Products. LCGC N. Am. 2022, 40, 27–31. [Google Scholar]
- Farsang, E.; Guillarme, D.; Veuthey, J.-L.; Beck, A.; Lauber, M.; Schmudlach, A.; Fekete, S. Coupling non-denaturing chromatography to mass spectrometry for the characterization of monoclonal antibodies and related products. J. Pharm. Biomed. Anal. 2020, 185, 113207. [Google Scholar] [CrossRef]
- Goyon, A.; Scott, B.; Crittenden, C.M.; Zhang, K. Analysis of pharmaceutical drug oligomers by selective comprehensive two-dimensional liquid chromatography-high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2022, 208, 114466. [Google Scholar] [CrossRef]
- Ji, S.; Wang, S.; Xu, H.; Su, Z.; Tang, D.; Qiao, X.; Ye, M. The application of on-line two-dimensional liquid chromatography (2DLC) in the chemical analysis of herbal medicines. J. Pharm. Biomed. Anal. 2018, 160, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Punt, A.M.; Langenhorst, J.B.; Egas, A.C.; Boelens, J.J.; van Kesteren, C.; van Maarseveen, E.M. Simultaneous quantification of busulfan, clofarabine and F-ARA-A using isotope labelled standards and standard addition in plasma by LC-MS/MS for exposure monitoring in hematopoietic cell transplantation conditioning. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1055–1056, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bei, E.; Shu, Y.; Li, S.; Liao, X.; Wang, J.; Zhang, X.; Chen, C.; Krasner, S. Occurrence of nitrosamines and their precursors in drinking water systems around mainland China. Water Res. 2016, 98, 168–175. [Google Scholar] [CrossRef]
- Zeng, T.; Plewa, M.J.; Mitch, W.A. N-Nitrosamines and halogenated disinfection byproducts in U.S. Full Advanced Treatment trains for potable reuse. Water Res. 2016, 101, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.M.; Hernandez, A.V. Ranitidine and Risk of N-Nitrosodimethylamine (NDMA) Formation. JAMA 2021, 326, 225–227. [Google Scholar] [CrossRef] [PubMed]


| Type | Specifications | Pore Size (Å) | Surface Area (m2/g) | pH Range | Capacity Factor a |
|---|---|---|---|---|---|
| Waters Atlantis T3 | 4.6 mm × 20 mm × 5.0 μm | 100 | 330 | 2.0–8.0 | 0.3 |
| Thermo RSLC | 3.0 mm × 33 mm × 3.0 μm | 120 | 300 | 1.5–10.0 | 0.4 |
| Waters Oasis Wax | 3.9 mm × 20 mm × 30 μm | 80 | 100 | 0–14.0 | 3.7 |
| Thermo PFP | 3.0 mm × 30 mm × 2.5 μm | 100 | 230 | 2.0–8.0 | 1.7 |
| Waters Oasis HLB | 3.9 mm × 20 mm × 5.0 μm | 80 | 786 | 0–14.0 | 6.0 |
| Left Pump | Right Pump | Valve Switch | |||||
|---|---|---|---|---|---|---|---|
| Time (min) | Flow Rate (mL/min) | Methanol (%) | Flow Rate (mL/min) | Acetonitrile (%) | Time (min) | Left Valve Position | Right Valve Position |
| 0 | 1.00 | 0 | 0.35 | 20 | 0 | 1–2 | 1–2 |
| 1.4 | 1.00 | 0 | 1.4 | 1–2 | 6–1 | ||
| 1.5 | 1.00 | 20 | 2.7 | 6–1 | 1–2 | ||
| 2.5 | 1.00 | 20 | 3.6 | 1–2 | 1–2 | ||
| 5.0 | 0.35 | 95 | |||||
| 12.5 | 0.35 | 95 | |||||
| 13.0 | 0.35 | 95 | |||||
| 17.0 | 1.00 | 0 | |||||
| 20.0 | 1.00 | 0 | |||||
| Compounds | Molecular Formula | Molecular Structure | Precursor m/z a | Quantifier m/z | Qualifier m/z | Collision Energy (eV) | Lens Offset |
|---|---|---|---|---|---|---|---|
| NDMA | C2H6N2O |  | 75.1 | 43.3 | 58.3 | 16 | 57.6 |
| NMEA | C3H8N2O |  | 89.0 | 88.5 | 43.3 | 5 | 125.1 |
| NPyr | C4H8N2O |  | 101.1 | 55.3 | 39.4 | 16 | 60.1 |
| NDEA | C4H10N2O |  | 103.1 | 75.2 | 27.5 | 11 | 57.6 |
| NPip | C5H10N2O |  | 115.1 | 69.2 | 41.4 | 15 | 63.1 |
| NMor | C4H8N2O2 |  | 117.1 | 87.2 | 86.2 | 13 | 58.1 |
| NDPA | C6H14N2O |  | 131.1 | 41.4 | 39.4 | 19 | 50.3 |
| NDBA | C8H18N2O |  | 159.1 | 41.4 | 39.4 | 37 | 58.3 |
| NDPhA | C12H10N2O |  | 199.0 | 169.1 | 66.2 | 10 | 56.1 |
| Analyte | Linearity Equation | Correlation coefficient (R2) | QC1 (10 ng/mL) | QC2 (50 ng/mL) | QC3 (100 ng/mL) | LOD b (ng/L) | LOQ c (ng/L) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD a(%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |||||
| NDMA | y = 7345 x + 13,183 | 0.9999 | 91.67 | 4.17 | 95.49 | 3.89 | 99.37 | 3.06 | 6.60 | 21.99 |
| NMEA | y = 108,885 x − 258,800 | 0.9998 | 97.78 | 3.55 | 98.02 | 1.93 | 100.7 | 1.63 | 6.14 | 20.46 |
| NPyr | y = 393,491 x − 1,238,308 | 0.9993 | 104.90 | 0.99 | 100.36 | 0.97 | 98.54 | 0.74 | 1.72 | 5.74 |
| NDEA | y = 256,265 x + 437,747 | 0.9998 | 93.48 | 2.67 | 105.88 | 2.50 | 96.36 | 1.87 | 0.86 | 2.87 |
| NPip | y = 732,010 x + 2,070,603 | 0.9994 | 95.37 | 2.79 | 96.54 | 3.85 | 101.0 | 1.30 | 2.70 | 8.99 |
| NMor | y = 46,808 x + 133,910 | 0.9994 | 99.43 | 3.80 | 98.07 | 2.99 | 100.2 | 2.18 | 2.14 | 7.12 |
| NDPA | y = 74,461 x + 164,445 | 0.9997 | 103.79 | 0.87 | 101.62 | 2.87 | 99.38 | 0.97 | 2.11 | 7.04 |
| NDBA | y = 20,982 x + 36,659 | 0.9998 | 96.85 | 1.24 | 103.87 | 3.48 | 98.73 | 2.68 | 3.29 | 10.98 |
| NDPhA | y = 816,649 x + 2,207,072 | 0.9995 | 100.86 | 1.01 | 99.45 | 0.76 | 98.95 | 0.68 | 0.12 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Li, Z.; Chen, H.; Xu, Y.; Jiang, X.; Du, E.; Lyu, Z.; Zheng, L.; Peng, M. An Online-SPE/SEC/LCMS Method for the Detection of N-Nitrosamine Disinfection Byproducts in Wastewater Plant Tailwater. Water 2022, 14, 2371. https://doi.org/10.3390/w14152371
Tang H, Li Z, Chen H, Xu Y, Jiang X, Du E, Lyu Z, Zheng L, Peng M. An Online-SPE/SEC/LCMS Method for the Detection of N-Nitrosamine Disinfection Byproducts in Wastewater Plant Tailwater. Water. 2022; 14(15):2371. https://doi.org/10.3390/w14152371
Chicago/Turabian StyleTang, Huili, Ziru Li, Hanqing Chen, Yang Xu, Xu Jiang, Erdeng Du, Zhen Lyu, Lu Zheng, and Mingguo Peng. 2022. "An Online-SPE/SEC/LCMS Method for the Detection of N-Nitrosamine Disinfection Byproducts in Wastewater Plant Tailwater" Water 14, no. 15: 2371. https://doi.org/10.3390/w14152371