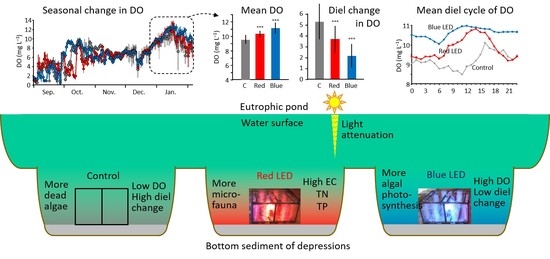
Improvement of Water Quality by Light-Emitting Diode Illumination at the Bottom of a Field Experimental Pond
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Pond
2.2. LED Devices
2.3. Experimental Period and Measurements
2.4. Statistical Analysis
3. Results
3.1. Overall Changes in Water Quality of the Pond during the Experiment
3.2. Differences in Water Quality of the Depressions in the Different Periods
3.3. The Diel Cycle of Water Quality and the Response to Rainfall
3.4. Nutrient Concentrations in the Depressions at the End of the Experiment
3.5. Periphyton and Phytoplankton Communities in the Depression at the End of the Experiment
4. Discussion
4.1. Differences in LED Effects among the Experimental Periods
4.2. Possible Algal and Microfaunal Activities in the Three Depressions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Group | Taxa | Control | Red-LED | Blue-LED |
|---|---|---|---|---|
| Cyanobacteria | Phormidium | 7 | ||
| Bacillariophyta | Achnanthes | 33 | 34 | |
| Cocconeis | 28 | 79 | 85 | |
| Diatoma | 16 | 2 | ||
| Gomphonema | 49 | |||
| Melosira varians | 1 | |||
| Rhoicosphenia | 14 | |||
| Other taxa | 15 | 12 | 13 | |
| Chlorophyta | Cladophora | 1 | ||
| Oedogonium | 9 | 1 | ||
| Protozoa | Vorticella | 3 | 36 | 22 |
| Group | Taxa | Control | Red-LED | Blue-LED |
|---|---|---|---|---|
| Cyanobacteria | Merismopedia | 7 | ||
| Bacillariophyta | Asterionella formosa | 3 | ||
| Aulacoseira distans | 2 | |||
| Achnanthes | 2 | |||
| Cyclotella | 1 | 11 | 3 | |
| Cymbella | 2 | |||
| Diatoma | 1 | |||
| Gomphonema acuminatum | 1 | |||
| Melosira varians | 3 | 1 | 1 | |
| Navicula | 3 | |||
| Nitzschia acicularis | 1 | 7 | ||
| Nitzschia palea | 1 | |||
| Nitzschia | 2 | |||
| Synedra acus | 1 | |||
| Synedra ulna | 4 | |||
| Other taxa | 1 | |||
| Chlorophyta | Chlorella | 1 | ||
| Oocystis | 3 | |||
| Scenedesmus | 4 | 8 | ||
| Other algae | Cryptomonas | 1 | ||
| Dinoflagellate | 4 | 1 | 8 | |
| Protozoa | Amoeba | 1 | ||
| Ciliata | 2 | |||
| Tintinnopsis | 1 | |||
| Rotifer | Keratella | 1 | ||
| Crustacea | Nauplius | 3 |
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xu, X.; Wang, M.; Wang, G.; Wu, S.; Li, Z.; Sun, H.; Shi, A.; Yang, Y. Climate warming and cyanobacteria blooms: Looks at their relationships from a new perspective. Water Res. 2017, 125, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Paerl, H.W.; Brookes, J.D.; Liu, J.; Jeppesen, E.; Zhu, G.; Zhang, Y.; Xu, H.; Shi, K.; Deng, J. Why Lake Taihu continues to be plagued with cyanobacterial blooms through 10 years (2007–2017) efforts. Sci. Bull. 2019, 64, 354–356. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, P.A.D.M.; Anderson, D.M.; Kaoru, Y.; White, A.W. The economic effects of harmful algal blooms in the United States: Estimates, assessment issues, and information needs. Estuaries 2002, 25, 819–837. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Paul, V.J. Global warming and cyanobacterial harmful algal blooms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; Volume 619, pp. 239–257. [Google Scholar]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Schelske, C.L.; Hodell, D.A. Using carbon isotopes of bulk sedimentary organic matter to reconstruct the history of nutrient loading and eutrophication in Lake Erie. Limnol. Oceanogr. 1995, 40, 918–929. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Van der Heijde, A.; Knijff, L.M.; Aalderink, R.H. Integrated modelling of eutrophication and organic contaminant fate & effects in aquatic ecosystems. A review. Water Res. 2001, 35, 3517–3536. [Google Scholar]
- Anderson, N.J.; Bennion, H.; Lotter, A.F. Lake eutrophication and its implications for organic carbon sequestration in Europe. Glob. Chang. Biol. 2014, 20, 2741–2751. [Google Scholar] [CrossRef] [Green Version]
- Gorham, E.; Boyce, F.M. Influence of lake surface area and depth upon thermal stratification and the depth of the summer thermocline. J. Great Lakes Res. 1989, 15, 233–245. [Google Scholar] [CrossRef]
- Foley, B.; Jones, I.D.; Maberly, S.C.; Rippey, B. Long-term changes in oxygen depletion in a small temperate lake: Effects of climate change and eutrophication. Freshw. Biol. 2012, 57, 278–289. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Dai, M.; Liu, J.; Kao, S.J.; Zhang, C.; Cai, W.J.; Wang, G.; Qian, W.; Zhao, M.; Sun, Z. Eutrophication-driven hypoxia in the East China Sea off the Changjiang Estuary. Environ. Sci. Technol. 2016, 50, 2255–2263. [Google Scholar] [CrossRef]
- Wetzel, R.G. The phosphorus cycle. In Limnology, 3rd ed.; Wetzel, R.G., Ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 239–288. [Google Scholar]
- Hupfer, M.; Lewandowski, J. Oxygen controls the phosphorus release from Lake Sediments—A long-lasting paradigm in limnology. Int. Rev. Hydrobiol. 2008, 93, 415–432. [Google Scholar] [CrossRef]
- Meyer-Reil, L.A.; Köster, M. Eutrophication of marine waters: Effects on benthic microbial communities. Mar. Pollut. Bull. 2000, 41, 255–263. [Google Scholar] [CrossRef]
- Kang, M.; Tian, Y.; Peng, S.; Wang, M. Effect of dissolved oxygen and nutrient levels on heavy metal contents and fractions in river surface sediments. Sci. Total Environ. 2019, 648, 861–870. [Google Scholar] [CrossRef]
- Beutel, M.W.; Leonard, T.M.; Dent, S.R.; Moore, B.C. Effects of aerobic and anaerobic conditions on P, N, Fe, Mn, and Hg accumulation in waters overlaying profundal sediments of an oligo-mesotrophic lake. Water Res. 2008, 42, 1953–1962. [Google Scholar] [CrossRef]
- Liem-Nguyen, V.; Skyllberg, U.; Björn, E. Thermodynamic modeling of the solubility and chemical speciation of mercury and methylmercury driven by organic thiols and micromolar sulfide concentrations in boreal wetland soils. Environ. Sci. Technol. 2017, 51, 3678–3686. [Google Scholar] [CrossRef]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef]
- Arend, K.K.; Beletsky, D.; DePinto, J.V.; Ludsin, S.A.; Roberts, J.J.; Rucinski, D.K.; Scavia, D.; Schwab, D.J.; Höök, T.O. Seasonal and interannual effects of hypoxia on fish habitat quality in central Lake Erie. Freshwat. Biol. 2011, 56, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, L.; Yan, Z.; Chao, C.; Yu, H.; Yu, D.; Liu, C. Effectiveness of dredging on internal phosphorus loading in a typical aquacultural lake. Sci. Total Environ. 2020, 744, 140883. [Google Scholar] [CrossRef] [PubMed]
- Beutel, M.W.; Horne, A.J. A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv. Manag. 1999, 15, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Bessa da Silva, M.; Gonçalves, F.; Pereira, R. Portuguese shallow eutrophic lakes: Evaluation under the Water Framework Directive and possible physicochemical restoration measures. Euro-Mediterr. J. Environ. Integr. 2019, 4, 3. [Google Scholar] [CrossRef]
- Hao, A.; Kobayashi, S.; Xia, D.; Mi, Q.; Yan, N.; Su, M.; Lin, A.; Zhao, M.; Iseri, Y. Controlling eutrophication via surface aerators in irregular-shaped urban ponds. Water 2021, 13, 3360. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Biswas, D.K.; Nian, Y.; Jiang, G. Potential of constructed wetlands in treating the eutrophic water: Evidence from Taihu Lake of China. Bioresour. Technol. 2008, 99, 1656–1663. [Google Scholar] [CrossRef]
- Zhao, F.; Xi, S.; Yang, X.; Yang, W.; Li, J.; Gu, B.; He, Z. Purifying eutrophic river waters with integrated floating island systems. Ecol. Eng. 2012, 40, 53–60. [Google Scholar] [CrossRef]
- Waajen, G.W.; Van Bruggen, N.C.; Pires, L.M.D.; Lengkeek, W.; Lürling, M. Biomanipulation with quagga mussels (Dreissena rostriformis bugensis) to control harmful algal blooms in eutrophic urban ponds. Ecol. Eng. 2016, 90, 141–150. [Google Scholar] [CrossRef]
- Keeling, P.J. The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu. Rev. Plant Biol. 2013, 64, 583–607. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Blanken, W.; Cuaresma, M.; Wijffels, R.H.; Janssen, M. Cultivation of microalgae on artificial light comes at a cost. Algal Res. 2013, 2, 333–340. [Google Scholar] [CrossRef]
- Schulze, P.S.; Barreira, L.A.; Pereira, H.G.; Perales, J.A.; Varela, J.C. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Kwon, H.K.; Oh, S.J.; Yang, H.S.; Kim, P.J. Phytoremediation by benthic microalgae (BMA) and light emitting diode (LED) in eutrophic coastal sediments. Ocean Sci. J. 2015, 50, 87–96. [Google Scholar] [CrossRef]
- Hao, A.; Kobayashi, S.; Yan, N.; Xia, D.; Zhao, M.; Iseri, Y. Improvement of water quality using a circulation device equipped with oxidation carriers and light emitting diodes in eutrophic pond mesocosms. J. Environ. Chem. Eng. 2021, 9, 105075. [Google Scholar] [CrossRef]
- Hao, A.; Yu, H.; Kobayashi, S.; Xia, D.; Zhao, M.; Iseri, Y. Effects of light-emitting diode illumination on sediment surface biological activities and releases of nutrients and metals to overlying water in eutrophic lake microcosms. Water 2022, 14, 1839. [Google Scholar] [CrossRef]
- Chang, W.Y.; Ouyang, H. Dynamics of dissolved oxygen and vertical circulation in fish ponds. Aquaculture 1988, 74, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.R.; Kragh, T.; Sand-Jensen, K. Extreme diel dissolved oxygen and carbon cycles in shallow vegetated lakes. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171427. [Google Scholar] [CrossRef]
- Boyd, C.E. Pond water aeration systems. Aquac. Eng. 1988, 18, 9–40. [Google Scholar] [CrossRef]
- Prokopkin, I.G.; Zadereev, E.S. A model study of the effect of weather forcing on the ecology of a meromictic Siberian lake. J. Oceanol. Limnol. 2018, 36, 2018–2032. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Photosynthesis as a function of the incident light. In Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Kirk, J.T.O., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 330–387. [Google Scholar]
- Langman, O.C.; Hanson, P.C.; Carpenter, S.R.; Hu, Y.H. Control of dissolved oxygen in northern temperate lakes over scales ranging from minutes to days. Aquat. Biol. 2010, 9, 193–202. [Google Scholar] [CrossRef]
- Sederias, J.; Colman, B. The interaction of light and low temperature on breaking the dormancy of Chara vulgaris oospores. Aquat. Bot. 2007, 87, 229–234. [Google Scholar] [CrossRef]
- Zou, W.; Wang, Z.; Song, Q.; Tang, S.; Peng, Y. Recruitment-promoting of dormant Microcystis aeruginosa by three benthic bacterial species. Harmful Algae 2018, 77, 18–28. [Google Scholar] [CrossRef]
- Hays, G.C. A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. In Migrations and Dispersal of Marine Organisms; Developments in Hydrobiology; Jones, M.B., Ingólfsson, A., Ólafsson, E., Helgason, G.V., Gunnarsson, K., Svavarsson, J., Eds.; Springer: Dordrecht, The Netherland, 2003; Volume 174, pp. 163–170. [Google Scholar]
- Williamson, C.E.; Fischer, J.M.; Bollens, S.M.; Overholt, E.P.; Breckenridge, J.K. Toward a more comprehensive theory of zooplankton diel vertical migration: Integrating ultraviolet radiation and water transparency into the biotic paradigm. Limnol. Oceanogr. 2011, 56, 1603–1623. [Google Scholar] [CrossRef] [Green Version]
- Martynova, D.M.; Gordeeva, A.V. Light-dependent behavior of abundant zooplankton species in the White Sea. J. Plankton Res. 2010, 32, 441–456. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iseri, Y.; Hao, A.; Haraguchi, T.; Oishi, T.; Kuba, T.; Asai, K.; Kobayashi, S. Improvement of Water Quality by Light-Emitting Diode Illumination at the Bottom of a Field Experimental Pond. Water 2022, 14, 2310. https://doi.org/10.3390/w14152310
Iseri Y, Hao A, Haraguchi T, Oishi T, Kuba T, Asai K, Kobayashi S. Improvement of Water Quality by Light-Emitting Diode Illumination at the Bottom of a Field Experimental Pond. Water. 2022; 14(15):2310. https://doi.org/10.3390/w14152310
Chicago/Turabian StyleIseri, Yasushi, Aimin Hao, Tomokazu Haraguchi, Tetsuya Oishi, Takahiro Kuba, Koji Asai, and Sohei Kobayashi. 2022. "Improvement of Water Quality by Light-Emitting Diode Illumination at the Bottom of a Field Experimental Pond" Water 14, no. 15: 2310. https://doi.org/10.3390/w14152310