Field Application of Spent Lime Water Treatment Residual for the Removal of Phosphorus and other Pollutants in Urban Stormwater Runoff
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Characterization of Spent Lime Drinking Water Treatment Residual
2.3. Design and Operation of Treatment System
2.4. Flow Monitoring and Water Quality Analysis
2.5. Contact Time
2.6. Toxicological Analysis
2.7. Pollutant Removal Assessment
3. Results
3.1. Characterization of Spent Lime DWTR
3.2. Storm Event Hydrology and Contact Time
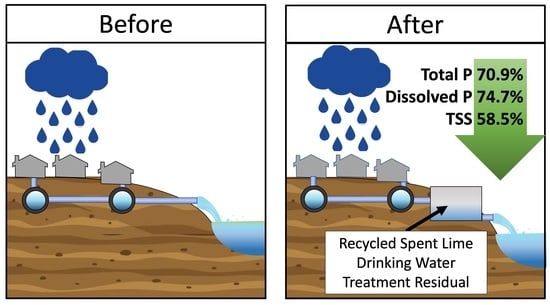
3.3. Removal of Phosphorus and Suspended Solids
3.4. Factors Potentially Associated with Phosphorus Removal
3.5. Metals Removal
3.6. Aquatic Toxicity
4. Discussion
4.1. Phosphorus Removal Performance
4.2. Pollutant Removal Mechanisms
4.3. Design Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A New Wine in an Old Bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jilbert, T.; Couture, R.-M.; Huser, B.J.; Salonen, K. Preface: Restoration of Eutrophic Lakes: Current Practices and Future Challenges. Hydrobiologia 2020, 847, 4343–4357. [Google Scholar] [CrossRef]
- Carpenter, S.R. Phosphorus Control Is Critical to Mitigating Eutrophication. Proc. Natl. Acad. Sci. USA 2008, 105, 11039–11040. [Google Scholar] [CrossRef] [Green Version]
- Douglas, G.B.; Hamilton, D.P.; Robb, M.S.; Pan, G.; Spears, B.M.; Lurling, M. Guiding Principles for the Development and Application of Solid-Phase Phosphorus Adsorbents for Freshwater Ecosystems. Aquat. Ecol. 2016, 50, 385–405. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The Pollution Conveyed by Urban Runoff: A Review of Sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef]
- Smith, J.S.; Winston, R.J.; Tirpak, R.A.; Wituszynski, D.M.; Boening, K.M.; Martin, J.F. The Seasonality of Nutrients and Sediment in Residential Stormwater Runoff: Implications for Nutrient-Sensitive Waters. J. Environ. Manag. 2020, 276, 111248. [Google Scholar] [CrossRef]
- Al-Ameri, M.; Hatt, B.; Le Coustumer, S.; Fletcher, T.; Payne, E.; Deletic, A. Accumulation of Heavy Metals in Stormwater Bioretention Media: A Field Study of Temporal and Spatial Variation. J. Hydrol. 2018, 567, 721–731. [Google Scholar] [CrossRef]
- Li, C.; Peng, C.; Chiang, P.-C.; Cai, Y.; Wang, X.; Yang, Z. Mechanisms and Applications of Green Infrastructure Practices for Stormwater Control: A Review. J. Hydrol. 2019, 568, 626–637. [Google Scholar] [CrossRef]
- Lintern, A.; McPhillips, L.; Winfrey, B.; Duncan, J.; Grady, C. Best Management Practices for Diffuse Nutrient Pollution: Wicked Problems Across Urban and Agricultural Watersheds. Environ. Sci. Technol. 2020, 54, 9159–9174. [Google Scholar] [CrossRef]
- Clary, J.; Jones, J.; Leisenring, M.; Strecker, E. International Stormwater BMP Database: 2020 Summary Statistics; The Water Research Foundation: Alexandria, VA, USA, 2020. [Google Scholar]
- Haygarth, P.M.; Jarvie, H.P.; Powers, S.M.; Sharpley, A.N.; Elser, J.J.; Shen, J.; Peterson, H.M.; Chan, N.-I.; Howden, N.J.K.; Burt, T.; et al. Sustainable Phosphorus Management and the Need for a Long-Term Perspective: The Legacy Hypothesis. Environ. Sci. Technol. 2014, 48, 8417–8419. [Google Scholar] [CrossRef]
- Lewis, W.M.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for Control of Anthropogenic Nitrogen and Phosphorus to Reduce Eutrophication of Inland Waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Okaikue-Woodi, F.E.K.; Cherukumilli, K.; Ray, J.R. A Critical Review of Contaminant Removal by Conventional and Emerging Media for Urban Stormwater Treatment in the United States. Water Res. 2020, 187, 116434. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J. The Past, Present, and Future of Phosphorus Removal Structures. Water 2021, 13, 797. [Google Scholar] [CrossRef]
- Penn, C.; Livingston, S.; Shedekar, V.; King, K.; Williams, M. Performance of Field-Scale Phosphorus Removal Structures Utilizing Steel Slag for Treatment of Subsurface Drainage. Water 2020, 12, 443. [Google Scholar] [CrossRef] [Green Version]
- Penn, C.J.; McGrath, J.M.; Rounds, E.; Fox, G.; Heeren, D. Trapping Phosphorus in Runoff with a Phosphorus Removal Structure. J. Environ. Qual. 2012, 41, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Habibiandehkordi, R.; Quinton, J.N.; Surridge, B.W.J. Effect of Equilibration Time on Estimates of the Maximum Phosphorus Sorption Capacity of Industrial By-Products Using the Langmuir Model. J. Soils Sediments 2014, 14, 1818–1828. [Google Scholar] [CrossRef]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A Review of Phosphorus Removal Structures: How to Assess and Compare Their Performance. Water 2017, 9, 583. [Google Scholar] [CrossRef]
- Muisa, N.; Nhapi, I.; Ruziwa, W.; Manyuchi, M.M. Utilization of Alum Sludge as Adsorbent for Phosphorus Removal in Municipal Wastewater: A Review. J. Water Process Eng. 2020, 35, 101187. [Google Scholar] [CrossRef]
- Turner, T.; Wheeler, R.; Stone, A.; Oliver, I. Potential Alternative Reuse Pathways for Water Treatment Residuals: Remaining Barriers and Questions—A Review. Water Air Soil Pollut. 2019, 230, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Nzihou, A.; Ren, B.; Lyczko, N.; Shen, C.; Kang, C.; Ji, B. Waterworks Sludge: An Underrated Material for Beneficial Reuse in Water and Environmental Engineering. Waste Biomass Valorization 2021, 12, 4239–4251. [Google Scholar] [CrossRef]
- Ament, M.R.; Roy, E.D.; Yongping, Y.; Hurley, S.E. Phosphorus Removal, Metals Dynamics, and Hydraulics in Stormwater Bioretention Systems Amended with Drinking Water Treatment Residuals. J. Sustain. Water Built Environ. 2022, 8, 04022003. [Google Scholar] [CrossRef]
- Vacca, K.; Komlos, J.; Wadzuk, B.M. Phosphorus Removal in Constructed Stormwater Wetland Mesocosms Amended with Water Treatment Residuals. Water Environ. Res. 2016, 88, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Al-Tahmazi, T.; Babatunde, A.O. Mechanistic Study of P Retention by Dewatered Waterworks Sludges. Environ. Technol. Innov. 2016, 6, 38–48. [Google Scholar] [CrossRef]
- Kelly Vargas, K.G.; Qi, Z. P Immobilizing Materials for Lake Internal Loading Control: A Review towards Future Developments. Crit. Rev. Environ. Sci. Technol. 2019, 49, 518–552. [Google Scholar] [CrossRef]
- Shrestha, P.; Salzl, M.T.; Jimenez, I.J.; Pradhan, N.; Hay, M.; Wallace, H.R.; Abrahamson, J.N.; Small, G.E. Efficacy of Spent Lime as a Soil Amendment for Nutrient Retention in Bioretention Green Stormwater Infrastructure. Water 2019, 11, 1575. [Google Scholar] [CrossRef] [Green Version]
- Stoner, D.; Penn, C.; McGrath, J.; Warren, J. Phosphorus Removal with By-Products in a Flow-Through Setting. J. Environ. Qual. 2012, 41, 654–663. [Google Scholar] [CrossRef]
- Szklarek, S.; Wagner, I.; Jurczak, T.; Zalewski, M. Sequential Sedimentation-Biofiltration System for the Purification of a Small Urban River (the Sokolowka, Lodz) Supplied by Stormwater. J. Environ. Manag. 2018, 205, 201–208. [Google Scholar] [CrossRef]
- Frątczak, W.; Michalska-Hejduk, D.; Zalewski, M.; Izydorczyk, K. Effective Phosphorous Reduction by a Riparian Plant Buffer Zone Enhanced with a Limestone-Based Barrier. Ecol. Eng. 2019, 130, 94–100. [Google Scholar] [CrossRef]
- Kirkkala, T.; Ventelä, A.-M.; Tarvainen, M. Long-Term Field-Scale Experiment on Using Lime Filters in an Agricultural Catchment. J. Environ. Qual. 2012, 41, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.M.; Penn, C.J.; Livingston, S.J. Utilization of Steel Slag in Blind Inlets for Dissolved Phosphorus Removal. Water 2020, 12, 1593. [Google Scholar] [CrossRef]
- Penn, C.; McGrath, J.; Bowen, J.; Wilson, S. Phosphorus Removal Structures: A Management Option for Legacy Phosphorus. J. Soil Water Conserv. 2014, 69, 51A–56A. [Google Scholar] [CrossRef]
- Jones, J.; Chang, N.-B.; Wanielista, M.P. Reliability Analysis of Nutrient Removal from Stormwater Runoff with Green Sorption Media under Varying Influent Conditions. Sci. Total Environ. 2015, 502, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Klimeski, A.; Uusitalo, R.; Turtola, E. Variations in Phosphorus Retention by a Solid Material While Scaling up Its Application. Environ. Technol. Innov. 2015, 4, 285–298. [Google Scholar] [CrossRef]
- Lyngsie, G.; Penn, C.J.; Pedersen, H.L.; Borggaard, O.K.; Hansen, H.C.B. Modelling of Phosphate Retention by Ca- and Fe-Rich Filter Materials under Flow-through Conditions. Ecol. Eng. 2015, 75, 93–102. [Google Scholar] [CrossRef]
- Ament, M.R.; Hurley, S.E.; Voorhees, M.; Perkins, E.; Yuan, Y.; Faulkner, J.W.; Roy, E.D. Balancing Hydraulic Control and Phosphorus Removal in Bioretention Media Amended with Drinking Water Treatment Residuals. ACS EST Water 2021, 1, 688–697. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms; US EPA: Washington, DC, USA, 2002.
- US EPA. A Plain English Guide to the EPA Part 503 Biosolids Rule; US EPA: Washington, DC, USA, 1994.
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Spears, B.M.; Maberly, S.C.; Pan, G.; Mackay, E.; Bruere, A.; Corker, N.; Douglas, G.; Egemose, S.; Hamilton, D.; Hatton-Ellis, T.; et al. Geo-Engineering in Lakes: A Crisis of Confidence? Environ. Sci. Technol. 2014, 48, 9977–9979. [Google Scholar] [CrossRef]
- Vadas, P.A.; Fiorellino, N.M.; Coale, F.J.; Kratochvil, R.; Mulkey, A.S.; McGrath, J.M. Estimating Legacy Soil Phosphorus Impacts on Phosphorus Loss in the Chesapeake Bay Watershed. J. Environ. Qual. 2018, 47, 480–486. [Google Scholar] [CrossRef]
- Inamdar, S.P.; Mostaghimi, S.; McClellan, P.W.; Brannan, K.M. BMP Impacts of Sediment and Nutrient Yields from an Agricultural Watershed in the Coastal Plain Region. Trans. ASAE 2001, 44, 1191. [Google Scholar] [CrossRef] [Green Version]
- Babatunde, A.O.; Zhao, Y.Q.; Zhao, X.H. Alum Sludge-Based Constructed Wetland System for Enhanced Removal of P and OM from Wastewater: Concept, Design and Performance Analysis. Bioresour. Technol. 2010, 101, 6576–6579. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wu, Y.; Bai, L.; Zhao, Y.; Yan, Z.; Jiang, H.; Liu, X. Recycling of Drinking Water Treatment Residue as an Additional Medium in Columns for Effective P Removal from Eutrophic Surface Water. J. Environ. Manag. 2018, 217, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kuster, A.C.; Huser, B.J.; Thongdamrongtham, S.; Padungthon, S.; Junggoth, R.; Kuster, A.T. Drinking Water Treatment Residual as a Ballast to Sink Microcystis Cyanobacteria and Inactivate Phosphorus in Tropical Lake Water. Water Res. 2021, 207, 117792. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Bryant, R.B.; Kleinman, P.J.A.; Allen, A.L. Removing Dissolved Phosphorus from Drainage Ditch Water with Phosphorus Sorbing Materials. J. Soil Water Conserv. 2007, 62, 269–276. [Google Scholar]
- Dayton, E.; Basta, N.T. A Method for Determining the Phosphorus Sorption Capacity and Amorphous Aluminum of Aluminum-Based Drinking Water Treatment Residuals. J. Environ. Qual. 2005, 34, 1112–1118. [Google Scholar] [CrossRef] [Green Version]
- Kuster, A.C.; Huser, B.J.; Padungthon, S.; Junggoth, R.; Kuster, A.T. Washing and Heat Treatment of Aluminum-Based Drinking Water Treatment Residuals to Optimize Phosphorus Sorption and Nitrogen Leaching: Considerations for Lake Restoration. Water 2021, 13, 2465. [Google Scholar] [CrossRef]
- Adhikari, R.A.; Bal Krishna, K.C.; Sarukkalige, R. Evaluation of Phosphorus Adsorption Capacity of Various Filter Materials from Aqueous Solution. Adsorpt. Sci. Technol. 2016, 34, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Engel, B.A.; Flanagan, D.C.; Gitau, M.W.; McMillan, S.K.; Chaubey, I. A Review on Effectiveness of Best Management Practices in Improving Hydrology and Water Quality: Needs and Opportunities. Sci. Total Environ. 2017, 601–602, 580–593. [Google Scholar] [CrossRef]
- Zarezadeh, V.; Lung, T.; Dorman, T.; Shipley, H.J.; Giacomoni, M. Assessing the Performance of Sand Filter Basins in Treating Urban Stormwater Runoff. Environ. Monit. Assess. 2018, 190, 697. [Google Scholar] [CrossRef]
- Jenkins, D.; Ferguson, J.F.; Menar, A.B. Chemical Processes for Phosphate Removal. Water Res. 1971, 5, 369–389. [Google Scholar] [CrossRef]
- Pilgrim, K.M.; Huser, B.J.; Brezonik, P.L. A Method for Comparative Evaluation of Whole-Lake and Inflow Alum Treatment. Water Res. 2007, 41, 1215–1224. [Google Scholar] [CrossRef]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Nutrients Removal from Urban Stormwater by Different Filter Materials. Water Air Soil Pollut. 2013, 225, 1778. [Google Scholar] [CrossRef]
- Wang, M.; Bai, S.; Wang, X. Enhanced Removal of Heavy Metals and Phosphate in Stormwater Filtration Systems Amended with Drinking Water Treatment Residual-Based Granules. J. Environ. Manag. 2021, 280, 111645. [Google Scholar] [CrossRef] [PubMed]
- Tunesi, S.; Poggi, V.; Gessa, C. Phosphate Adsorption and Precipitation in Calcareous Soils: The Role of Calcium Ions in Solution and Carbonate Minerals. Nutr. Cycl. Agroecosystems 1999, 53, 219–227. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A.; Heil, D.M.; Chandler, J.P.; Redente, E.F. Phosphorus Retention Mechanisms of a Water Treatment Residual. J. Environ. Qual. 2003, 32, 1857–1864. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristics and Mechanisms of Phosphate Adsorption on Dewatered Alum Sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Lee, L.Y.; Lim, F.Y.; Lyu, Z.; Zhu, H.; Ong, S.L.; Hu, J. Water Treatment Residual: A Critical Review of Its Applications on Pollutant Removal from Stormwater Runoff and Future Perspectives. J. Environ. Manag. 2020, 259, 109649. [Google Scholar] [CrossRef]
- Davis, A.P.; Burns, M. Evaluation of Lead Concentration in Runoff from Painted Structures. Water Res. 1999, 33, 2949–2958. [Google Scholar] [CrossRef]
- Godelitsas, A.; Astilleros, J.M.; Hallam, K.; Harissopoulos, S.; Putnis, A. Interaction of Calcium Carbonates with Lead in Aqueous Solutions. Environ. Sci. Technol. 2003, 37, 3351–3360. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Removal of Heavy Metals from Urban Stormwater Runoff Using Different Filter Materials. J. Environ. Chem. Eng. 2014, 2, 282–292. [Google Scholar] [CrossRef]
- Sauvé, S.; McBride, M.B.; Norvell, W.A.; Hendershot, W.H. Copper Solubility and Speciation of In Situ Contaminated Soils: Effects of Copper Level, PH and Organic Matter. Water Air Soil Pollut. 1997, 100, 133–149. [Google Scholar] [CrossRef]
- Yan, Q.; James, B.R.; Davis, A.P. Bioretention Media for Enhanced Permeability and Phosphorus Sorption from Synthetic Urban Stormwater. J. Sustain. Water Built Environ. 2018, 4, 4017013. [Google Scholar] [CrossRef] [Green Version]
- Claveau-Mallet, D.; Comeau, Y. Chemical Clogging and Evolution of Head Losses in Steel Slag Filters Used for Phosphorus Removal. Water 2020, 12, 1517. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Richards, B.K.; Medrano, P.A.; DeGroot, M.; Steenhuis, T.S. Dissolved Phosphorus from Undisturbed Soil Cores. Soil Sci. Soc. Am. J. 2003, 67, 458–470. [Google Scholar] [CrossRef]
- Penn, C.J.; Frankenberger, J.; Livingston, S. Introduction to P-TRAP Software for Designing Phosphorus Removal Structures. Agric. Environ. Lett. 2021, 6, e20043. [Google Scholar] [CrossRef]






| Parameter | Concentration (mg/kg Dry Weight) | Parameter | Concentration (mg/kg Dry Weight) |
|---|---|---|---|
| % Moisture | 59.3 | Magnesium | 35,700 |
| Aluminum | 5350 | Manganese | 115 |
| Ammonia as N | 74.4 | Mercury | <0.046 |
| Arsenic | <10.9 | Molybdenum | <8.2 |
| Barium | 122 | Nickel | 116 |
| Boron | <81.6 | Nitrate as N | <7.4 |
| Cadmium | <1.6 | Total Phosphorus | 117 |
| Calcium | 333,000 | Potassium | <1350 |
| Chloride | 113 | Selenium | <8.2 |
| Chromium | 184 | Silver | <5.4 |
| Copper | 15.3 | Sodium | <533 |
| Cyanide | <1.3 | Sulfate | <98.9 |
| Iron | 5080 | TKN | 831 |
| Lead | <10.9 | Zinc | <10.9 |
| Parameter | Influent Flow-Weighted Avg. (Range) 1,2 | Effluent Flow-Weighted Avg. (Range) 1,2 | Flow-Weighted % Removal (Range) 1,3 | p-Value 4 | Sampled Storm Events | Sampled Flow (m3) |
|---|---|---|---|---|---|---|
| Total P (mg/L) | 0.36 (0.096–2.7) | 0.11 (0.14–0.71) | 70.9% (8.3–94.5) | ≪0.001 *** | 35 | 1369 |
| Dissolved Reactive P (mg/L) | 0.12 (0.011–0.71) | 0.026 (0.001–0.064) | 78.5% (22.0–99.9) | ≪0.001 *** | 35 | 1369 |
| Total Dissolved P (mg/L) | 0.15 (0.14–0.34) | 0.038 (0.014–0.091) | 74.7% (−5.8–95.6) | 0.008 ** | 10 | 452 |
| TSS (mg/L) | 92.4 (12–350) | 38.0 (4.0–180) | 58.8% (−66.7–97.9) | ≪0.001 *** | 35 | 1369 |
| Chloride (mg/L) | 4.1 (0.42–29) | 5.9 (1.6–58.1) | −45.6% (−733–42.7) | 0.018 * | 33 | 1312 |
| Aluminum (mg/L) | 3.2 (0.33–8.5) | 1.6 (0.11–4.0) | 50.6% (4.0–91) | 0.014 * | 11 | 530 |
| Calcium (mg/L) | 11 (3.6–16) | 8.2 (2.6–18) | 28.1% (−12–65) | 0.13 | 11 | 530 |
| Iron (mg/L) | 3.7 (0.45–9.1) | 1.7 (0.12–4.3) | 54.9% (13–93) | <0.001 *** | 11 | 530 |
| Copper (µg/L) | 23 (8.7–29) | 14 (2.5–24) | 38.3% (−101–88) | n/a | 4 | 213 |
| Lead (µg/L) | 9.7 (3.1–12) | 2.4 (0.50–3.8) | 75.5% (−13–94) | n/a | 4 | 213 |
| Zinc (µg/L) | 108 (57–140) | 28 (6.6–69) | 74.3% (−22–94) | n/a | 4 | 213 |
| pH 5 | 6.09 (5.19–7.08) | 8.02 (7.15–9.52) | --- | --- | 8 † | 454 |
| Sampling Date | Water Source | % Survival 1 | Avg. Young (Range) 2 | pH Range | Hardness (mg/L as CaCO3) | Alkalinity (mg/L as CaCO3) | Specific Conductance (µS/cm) |
|---|---|---|---|---|---|---|---|
| 21 May 2012 | Lab Control | 90 | 18.7 (7–22) | 6.64–8.20 | 104 | 88 | 227 |
| Stormwater In | 100 | 20.3 (15–25) | 6.14–7.41 | 20 | 8 | 95 | |
| Effluent Out | 100 | 25.9 (21–29) | 6.66–8.02 | 128 | 92 | 576 | |
| 19 June 2012 | Lab Control | 100 | 17.7 (7–27) | 6.92–8.26 | 112 | 84 | 222 |
| Stormwater In | 100 | 26.9 (22–29) | 6.37–7.45 | 8 | 24 | 42 | |
| Effluent Out | 90 | 24.8 (13–30) | 6.97–7.87 | 116 | 108 | 469 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuster, A.C.; Pilgrim, K.M.; Kuster, A.T.; Huser, B.J. Field Application of Spent Lime Water Treatment Residual for the Removal of Phosphorus and other Pollutants in Urban Stormwater Runoff. Water 2022, 14, 2135. https://doi.org/10.3390/w14132135
Kuster AC, Pilgrim KM, Kuster AT, Huser BJ. Field Application of Spent Lime Water Treatment Residual for the Removal of Phosphorus and other Pollutants in Urban Stormwater Runoff. Water. 2022; 14(13):2135. https://doi.org/10.3390/w14132135
Chicago/Turabian StyleKuster, Anthony C., Keith M. Pilgrim, Anootnara T. Kuster, and Brian J. Huser. 2022. "Field Application of Spent Lime Water Treatment Residual for the Removal of Phosphorus and other Pollutants in Urban Stormwater Runoff" Water 14, no. 13: 2135. https://doi.org/10.3390/w14132135