River–Groundwater Interaction and Recharge Effects on Microplastics Contamination of Groundwater in Confined Alluvial Aquifers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Acquisition and Microplastics Sampling
2.3. Microplastics Extraction and Processing
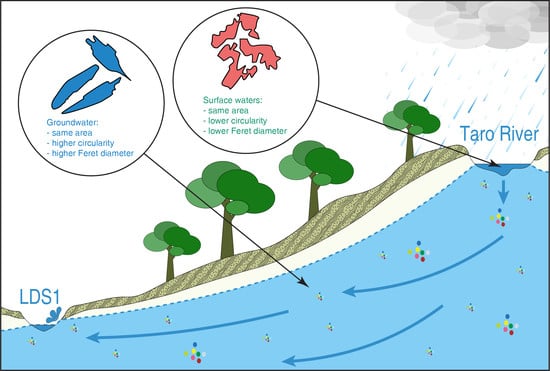
3. Results
3.1. Hydrogeological Features of the Study Area and the Effects of Aquifer Recharge
3.2. Microplastics Quantification and Geometric Characterization
4. Discussion
4.1. Assets and Disadvantages of the Extraction Protocol
4.2. Aquifer Recharge and River–Groundwater Interaction as Drivers of the Microplastic Contamination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer Nature: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Arthur, C.; Baker, J.; Bamford, H. International research workshop on the occurrence, effects, and fate of microplastic marine debris. In Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008. [Google Scholar]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Plastic scrubbers’ in hand cleansers: A further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 1996, 32, 867–871. [Google Scholar] [CrossRef]
- Zitko, V.; Hanlon, M. Another source of pollution by plastics: Skin cleaners with plastic scrubbers. Mar. Pollut. Bull. 1991, 22, 41–42. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic--an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olson, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Wei, X.-F.; Bohlén, M.; Lindblad, C.; Hedenqvist, M.; Hakonen, A. Microplastics generated from a biodegradable plastic in freshwater and seawater. Water Res. 2021, 198, 117123. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Colton Jr, J.B.; Knapp, F.D.; Burns, B.R. Plastic particles in surface waters of the Northwestern Atlantic. Science 1974, 185, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [Green Version]
- Schmoll, O.; Howard, G.; Chilton, J.; Chorus, I.; World Health Organization; Water, Sanitation and Health Team. Protecting Groundwater for Health: Managing the Quality of Drinking-Water Sources; Schmoll, O., Howard, G., Chilton, J., Chorus, I., Eds.; IWA Publishing: London, UK, 2006. [Google Scholar]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Hoellein, T.J.; Baranski, E.L. Microplastic Contamination in Karst Groundwater Systems. Groundwater 2019, 57, 189–196. [Google Scholar] [CrossRef]
- Goeppert, N.; Goldscheider, N. Experimental field evidence for transport of microplastic tracers over large distances in an alluvial aquifer. J. Hazard. Mater. 2021, 408, 124844. [Google Scholar] [CrossRef]
- Samandra, S.; Johnston, J.M.; Jaeger, J.E.; Symons, B.; Xie, S.; Currell, M.; Ellis, A.V.; Clarke, B.O. Microplastic contamination of an unconfined groundwater aquifer in Victoria, Australia. Sci. Total Environ. 2022, 802, 149727. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5, 17988. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Xu, P.; Zhu, B.; Bai, M.; Li, D. Microplastics in freshwater river sediments in Shanghai, China: A case study of risk assessment in mega-cities. Environ. Pollut. 2018, 234, 448–456. [Google Scholar] [CrossRef]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jia, Q.; Zhao, X.; Li, L.; Nie, Y.; Liu, H.; Ye, J. The occurrence of microplastics in water bodies in urban agglomerations: Impacts of drainage system overflow in wet weather, catchment land-uses, and environmental management practices. Water Res. 2020, 183, 116073. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Foschi, E.; D’Addato, F.; Bonoli, A. Plastic waste management: A comprehensive analysis of the current status to set up an after-use plastic strategy in Emilia-Romagna Region (Italy). Environ. Sci. Pollut. Res. 2021, 28, 24328–24341. [Google Scholar] [CrossRef]
- Zanini, A.; Petrella, E.; Sanangelantoni, A.M.; Angelo, L.; Ventosi, B.; Viani, L.; Rizzo, P.; Remelli, S.; Bartoli, M.; Bolpagni, R.; et al. Groundwater characterization from an ecological and human perspective: An interdisciplinary approach in the Functional Urban Area of Parma, Italy. Rend. Lincei. Sci. Fis. E Nat. 2019, 30, 93–108. [Google Scholar] [CrossRef]
- Viani, L. Idrodinamica Sotterranea Dell’acquifero Eterogeneo nel Parmense, Emilia-Romagna. Master’s Thesis, University of Parma, Parma, Italy, 2017. [Google Scholar]
- Lancini, J. Studio IDROGEOLOGICO in Area di Discarica: Il CASO del sito “Area Vasta di Viarolo” (PR). Master’s Thesis, University of Parma, Parma, Italy, 2019. [Google Scholar]
- Geognostic Tests Database of the Emilia-Romagna Region. 2020. Available online: https://ambiente.regione.emilia-romagna.it/it/geologia/cartografia/webgis-banchedati/banca-dati-geognostica (accessed on 23 February 2021).
- Petrucci, F.; Bigi, B.; Morestori, L.; Panicieri, E.; Pecorari, M.; Valloni, R. Ricerca C.N.R. Sulle Falde Acquifere Profonde Della Pianura Padana: Provv. di Parma e Piacenza (Destra T. Nure) Dell’istituto di Ricerca Sulle Acque—I.R.S.A.; Istituto di Ricerca Sulle Acque—I.R.S.A.: Roma, Italy, 1975; Volume 1, p. 306. [Google Scholar]
- Geological map of the Emilia-Romagna Region. 2004. Available online: https://ambiente.regione.emilia-romagna.it/it/geologia/cartografia/webgis-banchedati/webgis (accessed on 23 February 2021).
- Francese, R.; Chelli, A.; Molinari, F.C.; Paini, M. Microzonazione Sismica: Relazione illustrativa. Studio di Microzonazione Sismica di II Livello del Comune di Parma; Regione Emilia Romagna: Emilia-Romagna, Italy, 2016; p. 61. [Google Scholar]
- Regional Environmental Protection Agency of Emilia-Romagna (ARPAE). Hydrometric Levels Dataset. Available online: https://simc.arpae.it/dext3r/ (accessed on 10 May 2021).
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Water and Environment News, Issue 3, April 1998; International Atomic Energy Agency: Vienna, Austria, 1998. [Google Scholar]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [Green Version]
- Gohla, J.; Bračun, S.; Gretschel, G.; Koblmüller, S.; Wagner, M.; Pacher, C. Potassium carbonate (K2CO3)—A cheap, non-toxic and high-density floating solution for microplastic isolation from beach sediments. Mar. Pollut. Bull. 2021, 170, 112618. [Google Scholar] [CrossRef]
- Labbe, A.B.; Bagshaw, C.R.; Uttal, L. Inexpensive Adaptations of Basic Microscopes for the Identification of Microplastic Contamination Using Polarization and Nile Red Fluorescence Detection. J. Chem. Educ. 2020, 97, 4026–4032. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Alves, J.R.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Major factors influencing the quantification of Nile Red stained microplastics and improved automatic quantification (MP-VAT 2.0). Sci. Total Environ. 2020, 719, 137498. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- David, V. Statistics in Environmental Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis, version 0.9.1.9000. 2021. Available online: https://github.com/droglenc/FSA (accessed on 9 February 2021).
- Helsel, D.R.; Hirsch, R.M.; Ryberg, K.R.; Archfield, S.A.; Gilroy, E.J. Statistical Methods in Water Resources; 4-A3; U.S. Geological Survey: Reston, VA, USA, 2020; p. 484. [Google Scholar]
- Calabrese, L.; Ceriani, A. Note Illustrative Della Carta Geologica D’italia Alla Scala 1:50.000: Foglio 181: Parma Nord; ISPRA—Istituto Superiore per la Protezione e la Ricerca Ambientale: Firenze, Italy, 2009; p. 76. [Google Scholar]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Frei, S.; Piehl, S.; Gilfedder, B.S.; Löder, M.G.J.; Krutzke, J.; Wilhelm, L.; Laforsch, C. Occurence of microplastics in the hyporheic zone of rivers. Sci. Rep. 2019, 9, 15256. [Google Scholar] [CrossRef] [Green Version]
- Pirard, E. Image Measurements. In Image Analysis, Sediments and Paleoenvironments; Francus, P., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2004; pp. 59–86. [Google Scholar]
- Crawford, A. Understanding Fire Histories: The Importance of Charcoal Morphology. Ph.D. Thesis, University of Exeter, Exeter, UK, 2015. [Google Scholar]
- Heidel, S.G. The progressive lag of sediment concentration with flood waves. Eos 1956, 37, 56–66. [Google Scholar] [CrossRef]
- Keller, A.S.; Jimenez-Martinez, J.; Mitrano, D.M. Transport of Nano- and Microplastic through Unsaturated Porous Media from Sewage Sludge Application. Environ. Sci. Technol. 2020, 54, 911–920. [Google Scholar] [CrossRef]
- Weiss, T.H.; Mills, A.L.; Hornberger, G.M.; Herman, J.S. Effect of Bacterial Cell Shape on Transport of Bacteria in Porous Media. Environ. Sci. Technol. 1995, 29, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lazouskaya, V.; He, Q.; Jin, Y. Effect of Particle Shape on Colloid Retention and Release in Saturated Porous Media. J. Environ. Qual. 2010, 39, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Seymour, M.B.; Chen, G.; Su, C.; Li, Y. Transport and retention of colloids in porous media: Does shape really matter? Environ. Sci. Technol. 2013, 47, 8391–8398. [Google Scholar] [CrossRef] [PubMed]
- Bizmark, N.; Schneider, J.; Priestley, R.D.; Datta, S.S. Multiscale dynamics of colloidal deposition and erosion in porous media. Sci. Adv. 2020, 6, eabc2530. [Google Scholar] [CrossRef]
- De Sutter, R.; Verhoeven, R.; Krein, A. Simulation of sediment transport during flood events: Laboratory work and field experiments. Hydrol. Sci. J. 2001, 46, 599–610. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, S.; Lv, X.; Gao, Y.; Ge, L. Global trends and prospects in microplastics research: A bibliometric analysis. J. Hazard. Mater. 2020, 400, 123110. [Google Scholar] [CrossRef]







| Date | Sample | t (°C) | O2 (mg/L) | O2 (%) | EC (μS/cm) | ORP (mV) | pH | 3H (TU) | δD‰ (vs V-SMOW) | δ18O‰ (vs V-SMOW) |
|---|---|---|---|---|---|---|---|---|---|---|
| 25 March 2021 | LDS1 | 14.0 | 2.99 | 29 | 586.1 | 89.8 | 7.6 | 7.06 | −45.10 | −7.44 |
| 25 March 2021 | LDCAMPUS | 16.0 | 8.51 | 87 | 893.0 | 201.5 | 7.3 | - | - | - |
| 25 March 2021 | LDC3 | 6.4 | 8.96 | 73 | 608.7 | 143.1 | 8 | - | - | - |
| 13 April 2021 | LDS1 | 14.0 | 2.08 | 20 | 577.5 | 154.9 | 7.3 | 9.30 | −43.80 | −7.42 |
| 13 April 2021 | LDC3 | 7.7 | 9.22 | 77 | 467.7 | 139.5 | 8 | - | - | - |
| 13 April 2021 | LDCAMPUS | 14.0 | 7.67 | 81 | 308.4 | 190.2 | 6.9 | - | - | - |
| 13 April 2021 | Precipitation | - | - | - | - | - | - | 10.00 | - | −13.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severini, E.; Ducci, L.; Sutti, A.; Robottom, S.; Sutti, S.; Celico, F. River–Groundwater Interaction and Recharge Effects on Microplastics Contamination of Groundwater in Confined Alluvial Aquifers. Water 2022, 14, 1913. https://doi.org/10.3390/w14121913
Severini E, Ducci L, Sutti A, Robottom S, Sutti S, Celico F. River–Groundwater Interaction and Recharge Effects on Microplastics Contamination of Groundwater in Confined Alluvial Aquifers. Water. 2022; 14(12):1913. https://doi.org/10.3390/w14121913
Chicago/Turabian StyleSeverini, Edoardo, Laura Ducci, Alessandra Sutti, Stuart Robottom, Sandro Sutti, and Fulvio Celico. 2022. "River–Groundwater Interaction and Recharge Effects on Microplastics Contamination of Groundwater in Confined Alluvial Aquifers" Water 14, no. 12: 1913. https://doi.org/10.3390/w14121913