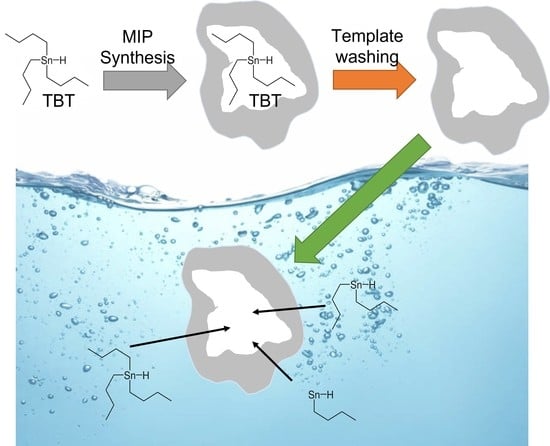
Synthesis of an Organotin Specific Molecularly Imprinted Polymer for Organotin Passive Sampling in Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of MIPs
2.2.1. Bulk Synthesis
2.2.2. Suspension Synthesis
2.2.3. Mini-Emulsion Synthesis
2.3. Washing Steps
2.4. Kinetics Experiments
2.5. Butyltin Species Analysis
3. Results
3.1. Synthetized MIP Comparisons
3.1.1. Bulk
3.1.2. Suspension
3.1.3. Mini-Emulsion
3.2. Template Molecule Removal
3.2.1. Purification of Bulk Polymers
3.2.2. Suspension and Mini-Emulsion Polymer Purification
3.3. Retention Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meador, J.P.; Krone, C.A.; Dyer, D.W.; Varanasi, U. Toxicity of sediment-associated tributyltin to infaunal invertebrates: Species comparison and the role of organic carbon. Mar. Environ. Res. 1997, 43, 219–241. [Google Scholar] [CrossRef]
- Alzieu, C. Environmental impact of TBT: The French experience. Sci. Total Env. 2000, 258, 99–102. [Google Scholar] [CrossRef]
- Hoch, M. Organotin compounds in the environment—An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Dobson, S.; Howe, P.; Floyd, P. Mono- and Disubstituted Methyltin, Butyltin, and Octyltin Compounds; World Health Organization: Geneva, Switzerland, 2006.
- Sousa, A.C.A.; Tanabe, S.; Pastorinho, M.R. Organotins: Sources and impacts on health and environment A2-Dellasala, Dominick A. In Encyclopedia of the Anthropocene; Goldstein, Elsevier: Oxford, UK, 2017; pp. 133–139. [Google Scholar]
- EPA 822-Z-99-001; Agency, U.S.E.P. National Recommended Water Quality Criteria. Office of Water 4304; 1999. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/20003OLV.PDF?Dockey=20003OLV.PDF (accessed on 25 January 2022).
- Directive 2008/105/EC of the European Parliament and of the Council (16 December 2008) on Environmental Quality Standards in the Field of Water Policy. Off. J. Eur. Union 2008, 348, 84–97. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM:l28180 (accessed on 20 February 2022).
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. Sources, Environmental Levels and Toxicity of Organotin in Marine Environment-A Review. Asian J. Chem. 2011, 23, 473–482. [Google Scholar]
- Langston, W.J.; Pope, N.D.; Davey, M.; Langston, K.M.; O’ Hara, S.C.M.; Gibbs, P.E.; Pascoe, P.L. Recovery from TBT pollution in English Channel environments: A problem solved? Mar. Pollut. Bull. 2015, 95, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.F.; Mills, G.A.; Parker, R.; Bolam, T.; Birchenough, A.; Kröger, S.; Fones, G.R. Trends in the analysis and monitoring of organotins in the aquatic environment. Trends Environ. Anal. Chem. 2015, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Martínez, R.; Palacios-Corvillo, M.A.; Greenwood, R.; Mills, G.A.; Vrana, B.; Gómez-Gómez, M.M. Calibration and use of the Chemcatcher® passive sampler for monitoring organotin compounds in water. Anal. Chim. Acta 2008, 618, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, R.; Mills, G.A.; Vrana, B.; Allan, I.; Aguilar-Martínez, R.; Morrison, G. Chapter 9 Monitoring of priority pollutants in water using chemcatcher passive sampling devices. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 48, pp. 199–229. [Google Scholar]
- Charriau, A.; Lissalde, S.; Poulier, G.; Mazzella, N.; Buzier, R.; Guibaud, G. Overview of the Chemcatcher(R) for the passive sampling of various pollutants in aquatic environments Part A: Principles, calibration, preparation and analysis of the sampler. Talanta 2016, 148, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Stepnowski, P.; Paszkiewicz, M. Pollutant analysis using passive samplers: Principles, sorbents, calibration and applications. A review. Environ. Chem. Lett. 2021, 19, 465–520. [Google Scholar] [CrossRef]
- MacKeown, H.; Benedetti, B.; Di Carro, M.; Magi, E. The study of polar emerging contaminants in seawater by passive sampling: A review. Chemosphere 2022, 299, 134448. [Google Scholar] [CrossRef] [PubMed]
- Folsvik, N.; Brevik, E.M.; Berge, J.A. Organotin compounds in a Norwegian fjord. A comparison of concentration levels in semipermeable membrane devices (SPMDs), blue mussels (Mytilus edulis) and water samples. J. Environ. Monit. 2002, 4, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Huckins, J.N.; Manuweera, G.K.; Petty, J.D.; Mackay, D.; Lebo, J.A. Lipid-containing semipermeable membrane devices for monitoring organic contaminants in water. Environ. Sci. Technol. 1993, 27, 2489–2496. [Google Scholar] [CrossRef]
- Guibal, R.; Buzier, R.; Charriau, A.; Lissalde, S.; Guibaud, G. Passive sampling of anionic pesticides using the Diffusive Gradients in Thin films technique (DGT). Anal. Chim. Acta 2017, 966, 1–10. [Google Scholar] [CrossRef]
- Casiot, C.; Egal, M.; Elbaz-Poulichet, F.; Bruneel, O.; Bancon-Montigny, C.; Cordier, M.-A.; Gomez, E.; Aliaume, C. Hydrological and geochemical control of metals and arsenic in a Mediterranean river contaminated by acid mine drainage (the Amous River, France); preliminary assessment of impacts on fish (Leuciscus cephalus). Appl. Geochem. 2009, 24, 787–799. [Google Scholar] [CrossRef]
- Davison, W.; Zhang, H.; Warnken, K.W. Chapter 16 Theory and applications of DGT measurements in soils and sediments. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 48, pp. 353–378. [Google Scholar]
- Branchet, P.; Cadot, E.; Sebag, D.; Fenet, H.; Ngatcha, B.N.; Gonzalez, C. POCIS evidence Yaoundé’s rivers pesticides contamination. In Goldschmidt 2017; European Association of Geochemistry: Aubière, France, 2017. [Google Scholar]
- Ibrahim, I. Study of the Applicability of Passive Samplers POCIS and Chemcatcher for Monitoring Pesticides in Aquatic Systems. Ecole Nationale Supérieure des Mines de Saint-Etienne. Available online: https://tel.archives-ouvertes.fr/tel-00864198/document (accessed on 10 January 2022).
- Alvarez, D.A.; Petty, J.D.; Huckins, J.N.; Jones-Lepp, T.L.; Getting, D.T.; Goddard, J.P.; Manahan, S.E. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ. Toxicol. Chem. 2004, 23, 1640–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrana, B.; Mills, G.A.; Dominiak, E.; Greenwood, R. Calibration of the Chemcatcher passive sampler for the monitoring of priority organic pollutants in water. Environ. Pollut. 2006, 142, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kingston, J.K.; Greenwood, R.; Mills, G.A.; Morrison, G.M.; Persson, L.B. Development of a novel passive sampling system for the time-averaged measurement of a range of organic pollutants in aquatic environments. J. Environ. Monit. 2000, 2, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.B.; Morrison, G.M.; Friemann, J.-U.; Kingston, J.; Mills, G.; Greenwood, R. Diffusional behaviour of metals in a passive sampling system for monitoring aquatic pollution. J. Environ. Monit. 2001, 3, 639–645. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, F.; Yang, T.; Gan, N.; Pan, D.; Cao, Y.; Wu, D. Synthesis and characterization of a molecularly imprinted polymer for the determination of trace tributyltin in seawater and seafood by liquid chromatography–tandem mass spectroscopy. J. Chromatogr. B 2013, 921–922, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K.; Muñoz-Olivas, R.; Cámara, C. A new polymeric adsorbent for screening and pre-concentration of organotin compounds in sediments and seawater samples. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 209–214. [Google Scholar] [CrossRef]
- Gallego-Gallegos, M.; Garrido, M.L.; Olivas, R.M.; Baravalle, P.; Baggiani, C.; Cámara, C. A new application of imprinted polymers: Speciation of organotin compounds. J. Chromatogr. A 2010, 1217, 3400–3407. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Gallegos, M.; Liva, M.; Olivas, R.M.; Cámara, C. Focused ultrasound and molecularly imprinted polymers: A new approach to organotin analysis in environmental samples. J. Chromatogr. A 2006, 1114, 82–88. [Google Scholar] [CrossRef]
- Gallego-Gallegos, M.; Muñoz-Olivas, R.; Cámara, C. Different formats of imprinted polymers for determining organotin compounds in environmental samples. J. Environ. Manag. 2009, 90, S69–S76. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Gallegos, M.; Muñoz-Olivas, R.; Martin-Esteban, A.; Cámara, C. Synthesis and evaluation of molecularly imprinted polymers for organotin compounds: A screening method for tributyltin detection in seawater. Anal. Chim. Acta 2005, 531, 33–39. [Google Scholar] [CrossRef]
- Ramström, O.; Mosbach, K. Synthesis and catalysis by molecularly imprinted materials. Curr. Opin. Chem. Biol. 1999, 3, 759–764. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for sample preparation: A review. Anal. Chim. Acta 2010, 668, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Puzio, K.; Claude, B.; Amalric, L.; Berho, C.; Grellet, E.; Bayoudh, S.; Nehmé, R.; Morin, P. Molecularly imprinted polymer dedicated to the extraction of glyphosate in natural waters. J. Chromatogr. A 2014, 1361, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Cao, X.; Zhang, Z.; Zhang, Z.; Jiang, Z.; Yin, J. Synthesis of molecularly imprinted polymer adsorbents for solid-phase extraction of strobilurin fungicides from agricultural products. J. Sep. Sci. 2020, 43, 2133–2141. [Google Scholar] [CrossRef]
- Haginaka, J. Monodispersed, molecularly imprinted polymers as affinity-based chromatography media. J. Chromatogr. B 2008, 866, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pichon, V.; Chapuis-Hugon, F. Role of molecularly imprinted polymers for selective determination of environmental pollutants—A review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Enzyme-like Catalysis by Molecularly Imprinted Polymers. Chem. Rev. 2002, 102, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Visnjevski, A.; Schomäcker, R.; Yilmaz, E.; Brüggemann, O. Catalysis of a Diels-Alder cycloaddition with differently fabricated molecularly imprinted polymers. Catal. Commun. 2005, 6, 601–606. [Google Scholar] [CrossRef]
- Mba Ekomo, V. From Electrochemical Molecularly Imprinted Polymer to Sensor: Feasibility Study for the Detection of Bisphenol A; Université de Toulon: Toulon, France, 2018; Available online: https://tel.archives-ouvertes.fr/tel-02273266/document (accessed on 10 January 2022).
- Zamora-Gálvez, A.; Mayorga-Matinez, C.C.; Parolo, C.; Pons, J.; Merkoçi, A. Magnetic nanoparticle-molecular imprinted polymer: A new impedimetric sensor for tributyltin detection. Electrochem. Commun. 2017, 82, 6–11. [Google Scholar] [CrossRef]
- Duda De Oliveira, D.; Rojas, E.G.; Antônio, M.; Fernandez, S. Should TBT continue to be considered an issue in dredging port areas? A brief review of the global evidence. Ocean. Coast. Manag. 2020, 197, 105303. [Google Scholar] [CrossRef]
- Berho, C.; Claude, B.; Coisy, E.; Togola, A.; Bayoudh, S.; Morin, P.; Amalric, L. Laboratory calibration of a POCIS-like sampler based on molecularly imprinted polymers for glyphosate and AMPA sampling in water. Anal. Bioanal. Chem. 2017, 409, 2029–2035. [Google Scholar] [CrossRef]
- Garnier, A.; Bancon-Montigny, C.; Delpoux, S.; Spinelli, S.; Avezac, M.; Gonzalez, C. Study of passive sampler calibration (Chemcatcher®) for environmental monitoring of organotin compounds: Matrix effect, concentration levels and laboratory vs in situ calibration. Talanta 2020, 219, 121316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiu, T.; Guo, L.; Ye, J.; He, L.; Li, X. The synthesis of molecular recognition polymer particles via miniemulsion polymerization. React. Funct. Polym. 2018, 126, 1–8. [Google Scholar] [CrossRef]
- Briant, N.; Bancon-Montigny, C.; Freydier, R.; Delpoux, S.; Elbaz-Poulichet, F. Behaviour of butyltin compounds in the sediment pore waters of a contaminated marina (Port Camargue, South of France). Chemosphere 2016, 150, 123–129. [Google Scholar] [CrossRef]
- Farrington, K.; Magner, E.; Regan, F. Predicting the performance of molecularly imprinted polymers: Selective extraction of caffeine by molecularly imprinted solid phase extraction. Anal. Chim. Acta 2006, 566, 60–68. [Google Scholar] [CrossRef]
- Navio, J.A.; Marchena, F.J.; Cerrillos, C.; Pablos, F. UV photolytic degradation of butyltin chlorides in water. J. Photochem. Photobiol. A Chem. 1993, 71, 97–102. [Google Scholar] [CrossRef]








| TBT (g) | MAA (µL) | EGDMA (mL) | AIBN (g) | ACN (mL) | PVA (g) | CH3Cl (mL) | Eau (mL) | SDS (g) | Hexadecane (g) | MIP Mass (g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulk 1 | 0.15 | 200 | 2 | 0.02 | 5 | ~2 | |||||
| Bulk 2 | 0.015 | 200 | 2 | 0.02 | 5 | ~2 | |||||
| Suspension 1 | 0.15 | 169 | 2 | 0.02 | 6 | 8 | 150 | ~2 | |||
| Suspension 2 | 0.15 | 169 | 2 | 0.02 | 0.6 | 8 | 150 | ~2 | |||
| Mini-emulsion | 0.15 | 169 | 2 | 0.02 | 50 | 0.015 | 0.5 | ~2 |
| MBT | DBT | TBT | |
|---|---|---|---|
| LOD ngSn·L−1 | 0.1 | 0.2 | 0.09 |
| LOQ ngSn·L−1 | 0.6 | 0.3 | 0.2 |
| Synthesis Mode | MBT (L·ng−1·s−1) | DBT (L·ng−1·s−1) | TBT (L·ng−1·s−1) |
|---|---|---|---|
| Mass (150 mg) | 3.9 10−7 | 4.3 10−5 | 1.3 10−6 |
| Mass (15 mg) | 7 10−7 | 3.2 10−5 | 1.3 10−6 |
| Suspension | 2.2 10−7 | 8.1 10−5 | 2.7 10−6 |
| Mini-emulsion | 6.2 10−8 | 4.3 10−7 | 9.6 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnier, A.; Montigny, C.; Causse, L.; Spinelli, S.; Avezac, M.; Otazaghine, B.; Gonzalez, C. Synthesis of an Organotin Specific Molecularly Imprinted Polymer for Organotin Passive Sampling in Seawater. Water 2022, 14, 1786. https://doi.org/10.3390/w14111786
Garnier A, Montigny C, Causse L, Spinelli S, Avezac M, Otazaghine B, Gonzalez C. Synthesis of an Organotin Specific Molecularly Imprinted Polymer for Organotin Passive Sampling in Seawater. Water. 2022; 14(11):1786. https://doi.org/10.3390/w14111786
Chicago/Turabian StyleGarnier, Antoine, Chrystelle Montigny, Léa Causse, Sylvie Spinelli, Murielle Avezac, Belkacem Otazaghine, and Catherine Gonzalez. 2022. "Synthesis of an Organotin Specific Molecularly Imprinted Polymer for Organotin Passive Sampling in Seawater" Water 14, no. 11: 1786. https://doi.org/10.3390/w14111786