Sustainable vs. Conventional Approach for Olive Oil Wastewater Management: A Review of the State of the Art
Abstract
:1. Introduction
2. OMW Composition
3. Environmental Impact of OMW
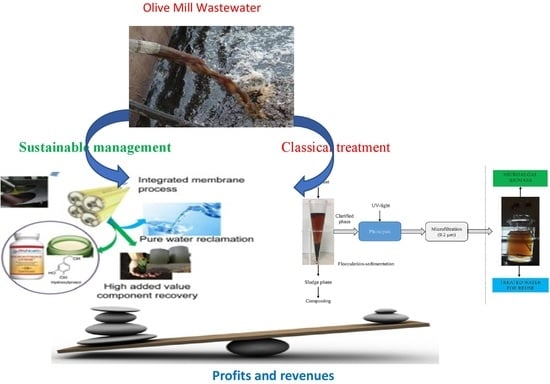
4. The Conventional Treatment Processes of OMW
4.1. Biological Based Processes
4.2. Physio-Chemical Treatments
4.3. Electrochemical Treatment Processes
4.4. Photocatalytic Degradation Methods
4.5. Advanced Oxidation Processes
4.6. Combined-Integrated Treatment Processes for OMW
5. Valorization Constituents of OMW and Sludge
5.1. Bio-Energy Materials
5.2. Bio-Chemical Materials
5.3. Medicinal Constituents of OMW
5.4. Agricultural Materials
5.5. Animal Feed and Food Materials
5.6. OMW Phenolic Compounds
6. Sustainable Recovery of Polyphenols from OMW
6.1. Polyphenols Extraction
6.2. Polyphenols Adsorption
6.3. Membranes Separation Processes
7. OMW Economical Separating Phenolic Compounds Study
8. Circular Economy
9. Concluding Remarks and Recommendations
- OMW is very complex wastewater containing toxic but valuable constituents that are of vital importance. This fact suggests the application of sustainable processes that can recover the valuable constituents from fresh OMW, treat the residual wastewater and reuse the final treated water.
- The required recovery processes should be mild and passive towards the chemical structure of the constituents, such as antioxidants and others. In other words, the recovery method should not be accompanied by any change in the chemical structure of the chemicals, which must not to lose their properties.
- According to the literature survey below, it seems that most research on OMW is from Mediterranean countries. However, such research is of a separate and fragmented nature, with little or no actual collaboration between researchers from different countries. This suggests the urgent need for donors to provide grants for relatively large projects that join researchers from different countries to achieve more successful and substantial results.
- Among the recovery methods, liquid–liquid or liquid–solid extraction using suitable selective solvents such as ethyl acetate and adsorption using selective adsorbent show a high percentage recovery of phenolic compounds from OMW. More research is needed to optimize these lab or pilot plant scale processes.
- Among the numerous research papers on this topic, only a few consider scaling up their experiment, such as Zagklis et al. [236]. More research should consider the large-scale application of single or combined recovery and treatment systems.
- One of the problems that usually faces OMW is that it is a seasonal phenomenon. This means that it appears in a specific period of the year that extends from October to January or February. This means that the fresh OMW is available only in this period, which puts pressure on the experiment’s teams. This fact encourages researchers to find a suitable OMW storage method that keeps it fresh with negligible degradation of the valuable constituents.
- More research could be performed to obtain the polyphenols and other valuable compounds from olive leaves.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuppala, S.; Bavasso, I.; Stoller, M.; Di Palma, L.; Vilardi, G. Olive mill wastewater integrated purification through pre-treatments using coagulants and biological methods: Experimental, modelling and scale-up. J. Clean. Prod. 2019, 236, 117622. [Google Scholar] [CrossRef]
- El Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Application of ultrafiltration for olive processing wastewaters treatment. J. Clean. Prod. 2014, 65, 432–438. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Abdul Raman, A.A. Energy intensified integrated advanced oxidation technology for the treatment of recalcitrant industrial wastewater. J. Clean. Prod. 2019, 206, 1025–1040. [Google Scholar] [CrossRef]
- Lin, S.; Mackey, H.; Hao, T.; Guo, G.; van Loosdrecht, M.C.; Chen, G. Biological sulfur oxidation in wastewater treatment: A review of emerging opportunities. Water Res. 2018, 143, 399–415. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Jamali, H.A.; Naghdali, Z.; Mousazadeh, M. Efficiency of electrocoagulation, sedimentation and filtration hybrid process in removing chemical oxygen demand and turbidity from carwash industrial wastewater: Optimization by response surface methodology. J. Maz. Univ. Med. Sci. 2019, 29, 106–120. [Google Scholar]
- Mousazadeh, M.; Niaragh, E.K.; Usman, M.; Khan, S.U.; Sandoval, M.A.; Al-Qodah, Z.; Bin Khalid, Z.; Gilhotra, V.; Emamjomeh, M.M. A critical review of state-of-the-art electrocoagulation technique applied to COD-rich industrial wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 43143–43172. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Cecconet, D.; Molognoni, D.; Capodaglio, A. Sustainable processing of dairy wastewater: Long-term pilot application of a bio-electrochemical system. J. Clean. Prod. 2018, 189, 563–569. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Foteinis, S.; Mantzavinos, D.; Tsoutsos, T. Life cycle assessment of advanced oxidation processes for olive mill wastewater treatment. J. Clean. Prod. 2013, 54, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, P.; Fernández, P.M.; Figueroa, L.I.C.; Pajot, H. Exploitation alternatives of olive mill wastewater: Production of value-added compounds useful for industry and agriculture. Biofuel Res. J. 2019, 6, 980–994. [Google Scholar] [CrossRef]
- Harwood, J. Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Ed.; Aspen Publishers, Inc.: Gaithersburg, MD, USA, 2013. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M.; Bani-Melhem, K.; Assirey, E.; Alananbeh, K.; Bouqellah, N. Biodegradation of olive mills wastewater using thermophilic bacteria. Desalination Water Treat. 2015, 56, 1908–1917. [Google Scholar] [CrossRef]
- D’Annibale, A.; Quaratino, D.; Federici, F.; Fenice, M. Effect of agitation and aeration on the reduction of pollutant load of olive mill wastewater by the white-rot fungus Panus tigrinus. Biochem. Eng. J. 2006, 29, 243–249. [Google Scholar] [CrossRef]
- Yesilada, O.; Sik, S.; Sam, M. Biodegradation of olive oil mill wastewaterby Coriolus versicolor and Funalia trogii: Effects of agitation, initial COD concentration, inoculum size and immobilization. World J. Microbiol. Biotechnol. 1998, 14, 37–42. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.-E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of olive mill wastewater into high-added value products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Davies, L.C.; Vilhena, A.M.; Novais, J.M.; Martins-Dias, S. Olive mill wastewater characteristics: Modelling and statistical analysis. Grasas Aceites 2004, 55, 233–241. [Google Scholar] [CrossRef]
- Benavente, V.; Fullana, A.; Berge, N.D. Life cycle analysis of hydrothermal carbonization of olive mill waste: Comparison with current management approaches. J. Clean. Prod. 2016, 142, 2637–2648. [Google Scholar] [CrossRef] [Green Version]
- Mekki, A.; Dhouib, A.; Aloui, F.; Sayadi, S. Olive wastewater as an ecological fertiliser. Agron. Sustain. Dev. 2006, 26, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Al-Qodah, Z.; Al-Bsoul, A.; Assirey, E.; Al-Shannag, M. Combined ultrasonic irradiation and aerobic biodegradation treatment for olive mills wastewaters. Environ. Eng. Manag. J. 2014, 13, 2109–2118. [Google Scholar]
- El Hanandeh, A. Energy recovery alternatives for the sustainable management of olive oil industry waste in Australia: Life cycle assessment. J. Clean. Prod. 2015, 91, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Stoller, M.; Ochando-Pulido, J.M.; Vilardi, G.; Vuppala, S.; Bravi, M.; Verdone, N.; Di Palma, L. Technical and economic impact of photocatalysis as a pretreatment process step in olive mill wastewater treatment by membranes. Chem. Eng. Trans. 2017, 57, 1171–1176. [Google Scholar]
- Haddar, W.; Baaka, N.; Meksi, N.; Elksibi, I.; Mhenni, M.F. Optimization of an ecofriendly dyeing process using the wastewater of the olive oil industry as natural dyes for acrylic fibres. J. Clean. Prod. 2014, 66, 546–554. [Google Scholar] [CrossRef]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Bio/Technol. 2007, 7, 1–26. [Google Scholar] [CrossRef]
- Halalsheh, M.; Kassab, G.; Shatanawi, K. Impact of legislation on olive mill wastewater management: Jordan as a case study. Water Policy 2021, 23, 343–357. [Google Scholar] [CrossRef]
- Chiavola, A.; Farabegoli, G.; Antonetti, F. Biological treatment of olive mill wastewater in a sequencing batch reactor. Biochem. Eng. J. 2014, 85, 71–78. [Google Scholar] [CrossRef]
- Capra, A.; Tamburino, V.; Zimbone, S.M. Irrigation systems for land spreading of olive oil mill wastewater. Terr. Aquat. Environ. Toxicol. 2010, 4, 65–74. [Google Scholar]
- Zagklis, D.P.; Arvaniti, E.C.; Papadakis, V.G.; Paraskeva, C.A. Sustainability analysis and benchmarking of olive mill wastewater treatment methods. J. Chem. Technol. Biotechnol. 2013, 88, 742–750. [Google Scholar] [CrossRef]
- Pulido, J.M.O. A review on the use of membrane technology and fouling control for olive mill wastewater treatment. Sci. Total Environ. 2015, 563–564, 664–675. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.; Perego, P. Kinetic and Isotherm Modelling of the Adsorption of Phenolic Compounds from Olive Mill Wastewater onto Activated Carbon. Food Technol. Biotechnol. 2015, 53, 207–214. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Arman, K.; Aghayani, E. Ecological risk assessment to Olive oil Mill Wastewater (OMW) with bioassay on plant species. Ecol. Environ. Conserv. 2014, 20, 229–234. [Google Scholar]
- Meftah, O.; Guergueb, Z.; Braham, M.; Sayadi, S.; Mekki, A. Long term effects of olive mill wastewaters application on soil properties and phenolic compounds migration under arid climate. Agric. Water Manag. 2018, 212, 119–125. [Google Scholar] [CrossRef]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental Effects Caused by Olive Mill Wastewaters: Toxicity Comparison of Low-Molecular-Weight Phenol Components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef]
- Al-Shaweesh, M.; Mohammed, M.; Al-Kabariti, D.; Khamash, D.; Al-Zawaidah, S.; Hindiyeh, M.; Omar, W. Olive mill wastewater (OMW) treatment by using ferric oxide dephenolization and chemical oxygen demand removal. Glob. NEST J. 2018, 20, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Jum’H, I.; Bani-Melhem, K. Treatment of olive mill effluent by adsorption on titanium oxide nanoparticles. Sci. Total Environ. 2019, 688, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Khdair, A.; Abu-Rumman, G.; Khdair, S. Pollution estimation from olive mills wastewater in Jordan. Heliyon 2019, 5, e02386. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Moreno, E.; Quevedo-Sarmiento, J.; Ramos-Cormenzana, A. Studies on antibacterial activity of waste waters from olive oil mills (alpechin): Inhibitory activity of phenolic and fatty acids. Chemosphere 1990, 20, 423–432. [Google Scholar] [CrossRef]
- Pérez, J.; Rubia, T.D.; Moreno, J.; Martínez, J. Phenolic content and antibacterial activity of olive oil waste waters. Environ. Toxicol. Chem. 1992, 11, 489–495. [Google Scholar] [CrossRef]
- Bonari, E.; Macchia, M.; Angelini, L.G.; Ceccarini, L. The waste waters from olive oil extraction: Their influence on the germinative characteristics of some cultivated and weed species. Agric. Mediterr. 1993, 123, 273–280. [Google Scholar]
- Greco, G.; Colarieti, M.L.; Toscano, G.; Iamarino, G.; Rao, M.A.; Gianfreda, L. Mitigation of Olive Mill Wastewater Toxicity. J. Agric. Food Chem. 2006, 54, 6776–6782. [Google Scholar] [CrossRef]
- Krogmeier, M.J.; Bremner, J.M. Effects of phenolic acids on seed germination and seedling growth in soil. Biol. Fertil. Soils 1989, 8, 116–122. [Google Scholar] [CrossRef]
- Boz, Ö.; Doğan, M.N.; Albay, F. Olive processing wastes for weed control. Weed Res. 2003, 43, 439–443. [Google Scholar] [CrossRef]
- Casa, R.; D’Annibale, A.; Pieruccetti, F.; Stazi, S.R.; Sermanni, G.G.; Cascio, B.L. Reduction of the phenolic components in olive-mill wastewater by an enzymatic treatment and its impact on durum wheat (Triticum durum Desf.) germinability. Chemosphere 2003, 50, 959–966. [Google Scholar] [CrossRef]
- El Hadrami, A. Physico-chemical Characterization and Effects of Olive Oil Mill Wastewaters Fertirrigation on the Growth of Some Mediterranean Crops. J. Agron. 2004, 3, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Quaratino, D.; D’Annibale, A.; Federici, F.; Cereti, C.F.; Rossini, F.; Fenice, M. Enzyme and fungal treatments and a combination thereof reduce olive mill wastewater phytotoxicity on Zea mays L. seeds. Chemosphere 2007, 66, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Garcia, J.L.; Ellouz, R. Integrated biological process for olive mill wastewater treatment. Bioprocess Biosyst. Eng. 1992, 8, 79–84. [Google Scholar] [CrossRef]
- Gunay, A.; Karadag, D. Recent developments in the anaerobic digestion of olive mill effluents. Process Biochem. 2015, 50, 1893–1903. [Google Scholar] [CrossRef]
- Ehaliotis, C.; Papadopoulou, K.; Kotsou, M.; Mari, I.; Balis, C. Adaptation and population dynamics of Azotobacter vinelandii during aerobic biological treatment of olive-mill wastewater. FEMS Microbiol. Ecol. 2000, 30, 301–311. [Google Scholar] [CrossRef]
- Oz, N.A.; Uzun, A.C. Ultrasound pretreatment for enhanced biogas production from olive mill wastewater. Ultrason. Sonochemistry 2014, 22, 565–572. [Google Scholar] [CrossRef]
- González, A.; Cuadros, F. Effect of aerobic pretreatment on anaerobic digestion of olive mill wastewater (OMWW): An ecoefficient treatment. Food Bioprod. Process. 2014, 95, 339–345. [Google Scholar] [CrossRef]
- Ruggeri, B.; Battista, F.; Bernardi, M.; Fino, D.; Mancini, G. The selection of pretreatment options for anaerobic digestion (AD): A case study in olive oil waste production. Chem. Eng. J. 2015, 259, 630–639. [Google Scholar] [CrossRef]
- Amaral, C.; Lucas, M.S.; Sampaio, A.; Peres, J.A.; Dias, A.A.; Peixoto, F.; Anjos, M.D.R.; Pais, C. Biodegradation of olive mill wastewaters by a wild isolate of Candida oleophila. Int. Biodeterior. Biodegradation 2012, 68, 45–50. [Google Scholar] [CrossRef]
- Sarika, R.; Kalogerakis, N.; Mantzavinos, D. Treatment of olive mill effluents: Part II. Complete removal of solids by direct flocculation with poly-electrolytes. Environ. Int. 2005, 31, 297–304. [Google Scholar] [CrossRef]
- Stoller, M. On the effect of flocculation as pretreatment process and particle size distribution for membrane fouling reduction. Desalination 2009, 240, 209–217. [Google Scholar] [CrossRef]
- Rizzo, L.; Lofrano, G.; Grassi, M.; Belgiorno, V. Pre-treatment of olive mill wastewater by chitosan coagulation and advanced oxidation processes. Sep. Purif. Technol. 2008, 63, 648–653. [Google Scholar] [CrossRef]
- Fragoso, R.A.; Duarte, E.D.A. Reuse of drinking water treatment sludge for olive oil mill wastewater treatment. Water Sci. Technol. 2012, 66, 887–894. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, F.; Badawy, M.; El-Khateeb, M.; El-Kalliny, A. Integrated treatment of olive mill wastewater (OMW) by the combination of Fenton’s reaction and anaerobic treatment. J. Hazard. Mater. 2008, 162, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Nieto, L.M.; Hodaifa, G.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Flocculation-Sedimentation Combined with Chemical Oxidation Process. Clean Soil Air Water 2011, 39, 949–955. [Google Scholar] [CrossRef]
- Alver, A.; Baştürk, E.; Kılıç, A.; Karataş, M. Use of advance oxidation process to improve the biodegradability of olive oil mill effluents. Process Saf. Environ. Prot. 2015, 98, 319–324. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Treatment of olive oil mill wastewater by combined process electro-Fenton reaction and anaerobic digestion. Water Res. 2006, 40, 2007–2016. [Google Scholar] [CrossRef]
- Ün, Ü.T.; Uğur, S.; Koparal, A.S.; Öğütveren, Ü.B. Electrocoagulation of olive mill waste waters. Sep. Purif. Technol. 2006, 52, 136–141. [Google Scholar]
- Un, U.T.; Altay, U.; Koparal, A.S.; Ogutveren, U.B. Complete Treatment of Olive Mill Wastewaters by Electrooxidation. Chem. Eng. J. 2008, 139, 445–452. [Google Scholar] [CrossRef]
- Cañizares, P.; Paz, R.; Saez, C.; Rodrigo, M.A. Costs of the electrochemical oxidation of wastewaters: A comparison with ozonation and Fenton oxidation processes. J. Environ. Manag. 2009, 90, 410–420. [Google Scholar] [CrossRef]
- Papastefanakis, N.; Mantzavinos, D.; Katsaounis, A. DSA electrochemical treatment of olive mill wastewater on Ti/RuO2 anode. J. Appl. Electrochem. 2010, 40, 729–737. [Google Scholar] [CrossRef]
- Kestioğlu, K.; Yonar, T.; Azbar, N. Feasibility of physico-chemical treatment and Advanced Oxidation Processes (AOPs) as a means of pretreatment of olive mill effluent (OME). Process Biochem. 2005, 40, 2409–2416. [Google Scholar] [CrossRef]
- Mert, B.K.; Yonar, T.; Kiliç, M.Y.; Kestioğlu, K. Pre-treatment studies on olive oil mill effluent using physicochemical, Fenton and Fenton-like oxidations processes. J. Hazard. Mater. 2010, 174, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Chatzisymeon, E.; Xekoukoulotakis, N.P.; Mantzavinos, D. Determination of key operating conditions for the photocatalytic treatment of olive mill wastewaters. Catal. Today 2009, 144, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Justino, C.I.; Duarte, K.; Loureiro, F.; Pereira, R.; Antunes, S.C.; Marques, S.M.; Gonçalves, F.; Rocha-Santos, T.A.; Freitas, A.C. Toxicity and organic content characterization of olive oil mill wastewater undergoing a sequential treatment with fungi and photo-Fenton oxidation. J. Hazard. Mater. 2009, 172, 1560–1572. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Hodaifa, G.; Ortega, M.D.V.; Martinez-Ferez, A. A Novel Photocatalyst with Ferromagnetic Core Used for the Treatment of Olive Oil Mill Effluents from Two-Phase Production Process. Sci. World J. 2013, 2013, 196470. [Google Scholar] [CrossRef] [Green Version]
- Ruzmanova, Y.; Stoller, M.; Chianese, A. Photocatalytic Treatment of Olive Mill Wastewater by Magnetic Core Titanium Dioxide Nanoparticles. Chem. Eng. Trans. 2013, 32, 2269–2274. [Google Scholar] [CrossRef]
- Bangkedphol, S.; Keenan, H.; Davidson, C.; Sakultantimetha, A.; Sirisaksoontorn, W.; Songsasen, A. Enhancement of tributyltin degradation under natural light by N-doped TiO2 photocatalyst. J. Hazard. Mater. 2010, 184, 533–537. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and Nitrogen Co-doped TiO2 with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Hu, S.; Wang, A.; Li, X.; Löwe, H. Hydrothermal synthesis of well-dispersed ultrafine N-doped TiO2 nanoparticles with enhanced photocatalytic activity under visible light. J. Phys. Chem. Solids 2010, 71, 156–162. [Google Scholar] [CrossRef]
- Papaphilippou, P.C.; Yiannapas, C.; Politi, M.; Daskalaki, V.M.; Michael, C.; Kalogerakis, N.; Mantzavinos, D.; Fatta-Kassinos, D. Sequential coagulation–flocculation, solvent extraction and photo-Fenton oxidation for the valorization and treatment of olive mill effluent. Chem. Eng. J. 2013, 224, 82–88. [Google Scholar] [CrossRef]
- Malvis, A.; Hodaifa, G.; Halioui, M.; Seyedsalehi, M.; Sánchez, S. Integrated process for olive oil mill wastewater treatment and its revalorization through the generation of high added value algal biomass. Water Res. 2019, 151, 332–342. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. On the Performance of Free Radicals Combined Electrocoagulation Treatment Processes. Sep. Purif. Rev. 2019, 48, 143–158. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.; Pimentel-Moral, S.; Verardo, V.; Martinez-Ferez, A. A focus on advanced physico-chemical processes for olive mill wastewater treatment. Sep. Purif. Technol. 2017, 179, 161–174. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; A Casas, J.; A Zazo, J.; Rodriguez, J.J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Badawy, M.; Ali, M.E.M. Fenton’s peroxidation and coagulation processes for the treatment of combined industrial and domestic wastewater. J. Hazard. Mater. 2006, 136, 961–966. [Google Scholar] [CrossRef]
- Badawy, M.I.; El Gohary, F.; Ghaly, M.Y.; Ali, M.E.M. Enhancement of olive mill wastewater biodegradation by homogeneous and heterogeneous photocatalytic oxidation. J. Hazard. Mater. 2009, 169, 673–679. [Google Scholar] [CrossRef]
- Minh, D.P.; Gallezot, P.; Azabou, S.; Sayadi, S.; Besson, M. Catalytic wet air oxidation of olive oil mill effluents: 4. Treatment and detoxification of real effluents. Appl. Catal. B Environ. 2008, 84, 749–757. [Google Scholar] [CrossRef]
- Azabou, S.; Najjar, W.; Bouaziz, M.; Ghorbel, A.; Sayadi, S. A compact process for the treatment of olive mill wastewater by combining wet hydrogen peroxide catalytic oxidation and biological techniques. J. Hazard. Mater. 2010, 183, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Nieto, L.M.; Hodaifa, G.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Degradation of organic matter in olive-oil mill wastewater through homogeneous Fenton-like reaction. Chem. Eng. J. 2011, 173, 503–510. [Google Scholar] [CrossRef]
- Hodaifa, G.; Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Martinez-Ferez, A. Optimization of continuous reactor at pilot scale for olive-oil mill wastewater treatment by Fenton-like process. Chem. Eng. J. 2013, 220, 117–124. [Google Scholar] [CrossRef]
- Nieto, L.M.; Hodaifa, G.; Vives, S.R.; Casares, J.A.G. Industrial Plant for Olive Mill Wastewater from Two-Phase Treatment by Chemical Oxidation. J. Environ. Eng. 2010, 136, 1309–1313. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Justino, C.; Marques, A.G.; Duarte, K.R.; Duarte, A.; Pereira, R.; Rocha-Santos, T.; Freitas, A.C. Degradation of phenols in olive oil mill wastewater by biological, enzymatic, and photo-Fenton oxidation. Environ. Sci. Pollut. Res. 2010, 17, 650–656. [Google Scholar] [CrossRef]
- Azabou, S.; Najjar, W.; Gargoubi, A.; Ghorbel, A.; Sayadi, S. Catalytic wet peroxide photo-oxidation of phenolic olive oil mill wastewater contaminants: Part II. Degradation and detoxification of low-molecular mass phenolic compounds in model and real effluent. Appl. Catal. B Environ. 2007, 77, 166–174. [Google Scholar] [CrossRef]
- Karageorgos, P.; Coz, A.; Charalabaki, M.; Kalogerakis, N.; Xekoukoulotakis, N.P.; Mantzavinos, D. Ozonation of weathered olive mill wastewaters. J. Chem. Technol. Biotechnol. 2006, 81, 1570–1576. [Google Scholar] [CrossRef]
- Farabegoli, G.; Chiavola, A.; Rolle, E. SBR treatment of olive mill wastewaters: Dilution or pre-treatment? Water Sci. Technol. 2012, 65, 1684–1691. [Google Scholar] [CrossRef]
- Martini, E.; Tomassetti, M.; Campanella, L.; Fortuna, A. Reducing the pollutant load of olive mill wastewater by photocatalytic membranes and monitoring the process using both tyrosinase biosensor and COD test. Front. Chem. 2013, 1, 36. [Google Scholar] [CrossRef] [Green Version]
- Amor, C.; Lucas, M.S.; García, J.; Dominguez, J.R.; De Heredia, J.B.; Peres, J.A. Combined treatment of olive mill wastewater by Fenton’s reagent and anaerobic biological process. J. Environ. Sci. Health Part A 2015, 50, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Salameh, W.K.B. Treatment of Olive mill Wastewater by ozonation and electrocoagulation processes. Civ. Environ. Res. 2015, 7, 80. [Google Scholar]
- Al-Bawab, A.; Alshawawreh, F.; Abu-Dalo, M.A.; Al-Rawashdeh, N.A.; Bozeya, A. Separation of soluble phenolic compounds from olive mill wastewater (OMW) using modified surfactant. Fresenius Environ. Bull. 2017, 26, 1949–1958. [Google Scholar]
- Kirmaci, A.; Duyar, A.; Akgul, V.; Akman, D.; Cirik, K. Optimization of Combined Ozone/Fenton Process on Olive Mill Wastewater Treatment. Aksaray Univ. J. Sci. Eng. 2018, 2, 52–62. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Jomaa, N.; Hourieh, Y. Integrated Process for the Treatment of Olive Oil Mill Waste Water (OMW). Chem. Res. J. 2020, 5, 121–129. [Google Scholar]
- Hattab, A.; Bagané, M.; Ben Amor, H. Combined Treatment by Coagulation-Flocculation and Oxidation of Olive Mill Wastewater. J. Mater. Environ. Sci. 2020, 11, 522–530. [Google Scholar]
- Gebreyohannes, A.Y.; Mazzei, R.; Lidietta, G. Trends and current practices of olive mill wastewater treatment. Sep. Purif. Technol. 2016, 162, 45–60. [Google Scholar] [CrossRef]
- Application of integrated membrane process and its future perspective. Water Environ. J. 2021, 35, 986–997.
- Benamar, A.; Mahjoubi, F.Z.; Barka, N.; Kzaiber, F.; Boutoial, K.; Ali, G.A.M.; Oussama, A. Olive mill wastewater treatment using infiltration percolation in column followed by aerobic biological treatment. SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ait-Hmane, A.; Mandi, L.; Ouazzani, N.; Hammou, H.A.; Hejjaj, A.; Alahiane, S.; Assabbane, A. Combined treatment of olive mill wastewater by multi-soil-layering ecotechnology and adsorption on activated carbon/lime. Desalination Water Treat. 2021, 233, 253–260. [Google Scholar] [CrossRef]
- Al-Rousan, D. Treatment of real olive mill wastewater by sole and combination of H2 O2, O3, and UVA: Effect of doses and ratios on organic content and biodegradability. Jordan J. Earth Environ. Sci. 2021, 12, 122–133. [Google Scholar]
- Shahamat, Y.D.; Khani, M.R.; Mahdizadeh, H.; Kannan, K.; Kalankesh, L.R.; Kamarehei, B.; Baneshi, M.M. Olive Mill Wastewater (OMW) Treatment by Hybrid Processes of Electrocoagulation/Catalytic Ozonation and Biodegradation. Environ. Eng. Manag. J. 2020, 19, 1401–1410. [Google Scholar] [CrossRef]
- Khedkar, R.; Singh, K. Food industry waste: A panacea or pollution hazard? In Paradigms in Pollution Prevention; Springer: Cham, Switzerland, 2018; pp. 35–47. [Google Scholar] [CrossRef]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Khoufi, S.; Feki, F.; Sayadi, S. Detoxification of olive mill wastewater by electrocoagulation and sedimentation processes. J. Hazard. Mater. 2007, 142, 58–67. [Google Scholar] [CrossRef]
- Bellou, S.; Makri, A.; Sarris, D.; Michos, K.; Rentoumi, P.; Celik, A.; Papanikolaou, S.; Aggelis, G. The olive mill wastewater as substrate for single cell oil production by Zygomycetes. J. Biotechnol. 2014, 170, 50–59. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Extraction of antioxidants from olive mill wastewater and electro-coagulation of exhausted fraction to reduce its toxicity on anaerobic digestion. J. Hazard. Mater. 2008, 151, 531–539. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Moraes, B.; Zaiat, M.; Bonomi, A. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives. Renew. Sustain. Energy Rev. 2015, 44, 888–903. [Google Scholar] [CrossRef]
- Fezzani, B.; Ben Cheikh, R. Two-phase anaerobic co-digestion of olive mill wastes in semi-continuous digesters at mesophilic temperature. Bioresour. Technol. 2010, 101, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, M.I.; Modallal, N. Ethanol Production from Olive Mill Wastewater (OMW) Pretreated with Pleurotus sajor-caju. Energy Fuels 2008, 22, 150–154. [Google Scholar] [CrossRef]
- Yousuf, A.; Sannino, F.; Addorisio, V.; Pirozzi, D. Microbial Conversion of Olive Oil Mill Wastewaters into Lipids Suitable for Biodiesel Production. J. Agric. Food Chem. 2010, 58, 8630–8635. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Gaitis, F.; Katsaris, P.; Skoulika, S.; Iliopoulos, N.; Zervakis, G.I. The effects of olives harvest period and production year on olive mill wastewater properties—Evaluation of Pleurotus strains as bioindicators of the effluent’s toxicity. Chemosphere 2013, 92, 399–405. [Google Scholar] [CrossRef]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautová, V.; Zervakis, G.I. Olive mill wastewater biodegradation potential of white-rot fungi – Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef]
- Cordova, J.; Nemmaoui, M.; Ismaïli-Alaoui, M.; Morin, A.; Roussos, S.; Raimbault, M.; Benjilali, B. Lipase production by solid state fermentation of olive cake and sugar cane bagasse. J. Mol. Catal. B Enzym. 1998, 5, 75–78. [Google Scholar] [CrossRef]
- Federici, F. Production, purification and partial characterization of an endopolygalacturonase fromCryptococcus albidus var.albidus. Antonie Leeuwenhoek 1985, 51, 139–150. [Google Scholar] [CrossRef]
- Petruccioli, M.; Servili, M.; Montedoro, G.F.; Federici, F. Development of a recycle procedure for the utilization of vegetation waters in the olive-oil extraction process. Biotechnol. Lett. 1988, 10, 55–60. [Google Scholar] [CrossRef]
- Sutherland, I.W. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998, 16, 41–46. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.; Ramos-Cormenzana, A. Xanthan production from olive-mill wastewaters. Int. Biodeterior. Biodegrad. 1996, 38, 263–270. [Google Scholar] [CrossRef]
- López, M.J.; Moreno, J.; Ramos-Cormenzana, A. Xanthomonas campestris strain selection for xanthan production from olive mill wastewaters. Water Res. 2001, 35, 1828–1830. [Google Scholar] [CrossRef]
- Ramos-Cormenzana, A.; Monteoliva-Sanchez, M.; Lopez, M.J. Bioremediation of alpechin. Int. Biodeterior. Biodegrad. 1995, 35, 249–268. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, M.; Monteoliva-Sánchez, M.; Suárez, A.; Guerra, V.; Lizama, C.; Bennasar, A.; Ramos-Cormenzana, A. Paenibacillus jamilae sp. nov., an exopolysaccharide-producing bacterium able to grow in olive-mill wastewater. Int. J. Syst. Evol. Microbiol. 2001, 51, 1687–1692. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Bravo, A.; Jimenez-Valera, M.; Moreno, E.; Guerra, V.; Ramos-Cormenzana, A. Biological Response Modifier Activity of an Exopolysaccharide from Paenibacillus jamilae CP-7. Clin. Diagn. Lab. Immunol. 2001, 8, 706–710. [Google Scholar] [CrossRef] [Green Version]
- Crognale, S.; Federici, F.; Petruccioli, M. β-Glucan production byBotryosphaeria rhodinaon undiluted olive-mill wastewaters. Biotechnol. Lett. 2004, 25, 2013–2015. [Google Scholar] [CrossRef]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive oil mill wastewater valorisation by fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Morillo, J.A.; del Águila, V.G.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Production and characterization of the exopolysaccharide produced by Paenibacillus jamilae grown on olive mill-waste waters. World J. Microbiol. Biotechnol. 2007, 23, 1705–1710. [Google Scholar] [CrossRef]
- Aguilera, M.; Quesada, M.T.; del Águila, V.G.; Morillo, J.A.; Rivadeneyra, M.A.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Characterisation of Paenibacillus jamilae strains that produce exopolysaccharide during growth on and detoxification of olive mill wastewaters. Bioresour. Technol. 2008, 99, 5640–5644. [Google Scholar] [CrossRef]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant Producing Microbes and their Potential Applications: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Mercade, E.; Manresa, A.; Robert, M.; Espuny, M.; de Andrés, C.; Guinea, J. Olive oil mill effluent (OOME). New substrate for biosurfactant production. Bioresour. Technol. 1993, 43, 1–6. [Google Scholar] [CrossRef]
- Colak, A.K.; Kahraman, H. The use of raw cheese whey and olive oil mill wastewater for rhamnolipid production by recombinant Pseudomonas aeruginosa. Environ. Exp. Biol. 2013, 11, 125–130. [Google Scholar]
- Ramírez, I.M.; Tsaousi, K.; Rudden, M.; Marchant, R.; Alameda, E.J.; García-Román, M.; Banat, I.M. Rhamnolipid and surfactin production from olive oil mill waste as sole carbon source. Bioresour. Technol. 2015, 198, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Salido, S.; Pérez-Bonilla, M.; Adams, R.P.; Altarejos, J. Phenolic Components and Antioxidant Activity of Wood Extracts from 10 Main Spanish Olive Cultivars. J. Agric. Food Chem. 2015, 63, 6493–6500. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef]
- Allouche, N.; Fki, A.I.; Sayadi, S. Toward a High Yield Recovery of Antioxidants and Purified Hydroxytyrosol from Olive Mill Wastewaters. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Scognamiglio, F. A simple thin layer chromatographic method to detect the main polyphenols occurring in olive oil vegetation waters. Phytochem. Anal. 1992, 3, 270–275. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Boráu, V.; García, I.; Jiménez, C.; Lafont, F.; María Marinas, J.; Urbano, F.J. Qualitative and Quantitative Analyses of Phenolic Compounds by High-performance Liquid Chromatography and Detection with Atmospheric Pressure Chemical Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 1585–1590. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- DellaGreca, M.; Previtera, L.; Temussi, F.; Zarrelli, A. Low-molecular-weight components of olive oil mill waste-waters. Phytochem. Anal. 2004, 15, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Ena, A.; Pintucci, C.; Carlozzi, P. The recovery of polyphenols from olive mill waste using two adsorbing vegetable matrices. J. Biotechnol. 2011, 157, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kalmıs, E.; Azbar, N.; Yıldız, H.; Kalyoncu, F. Feasibility of using olive mill effluent (OME) as a wetting agent during the cultivation of oyster mushroom, Pleurotus ostreatus, on wheat straw. Bioresour. Technol. 2008, 99, 164–169. [Google Scholar] [CrossRef]
- Altieri, R.; Esposito, A.; Parati, F.; Lobianco, A.; Pepi, M. Performance of olive mill solid waste as a constituent of the substrate in commercial cultivation of Agaricus bisporus. Int. Biodeterior. Biodegrad. 2009, 63, 993–997. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.C.; Zervakis, G.I. Detoxification of Olive Mill Wastewater and Bioconversion of Olive Crop Residues into High-Value-Added Biomass by the Choice Edible Mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Bertin, L.; Ferri, F.; Scoma, A.; Marchetti, L.; Fava, F. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem. Eng. J. 2011, 171, 1287–1293. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Effect of Processing Conditions, Prestorage Treatment, and Storage Conditions on the Phenol Content and Antioxidant Activity of Olive Mill Waste. J. Agric. Food Chem. 2008, 56, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Romero, M.-P.; Ramo, T.; Macià, A.; Motilva, M.-J. Methods for Preparing Phenolic Extracts from Olive Cake for Potential Application as Food Antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef] [PubMed]
- Bedouhene, S.; Hurtado-Nedelec, M.; Sennani, N.; Marie, J.-C.; El-Benna, J.; Moulti-Mati, F. Polyphenols Extracted from Olive Mill Wastewater Exert a Strong Antioxidant Effect in Human Neutrophils. Int. J. Waste Resour. 2014, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Fiore, A.; Fogliano, V.; Morales, F.J. Correction: Carbonyl trapping and antiglycative activities of olive oil mill wastewater. Food Funct. 2015, 6, 3399. [Google Scholar] [CrossRef] [Green Version]
- Albini, A.; Festa, M.M.G.; Ring, N.; Baci, D.; Rehman, M.; Finzi, G.; Sessa, F.; Zacchigna, S.; Bruno, A.; Noonan, D.M. A Polyphenol-Rich Extract of Olive Mill Wastewater Enhances Cancer Chemotherapy Effects, While Mitigating Cardiac Toxicity. Front. Pharmacol. 2021, 12, 1977. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Cassano, A. Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds. Antioxidants 2020, 9, 602. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Mekki, A.; Dhouib, A.; Sayadi, S. Review: Effects of olive mill wastewater application on soil properties and plants growth. Int. J. Recycl. Org. Waste Agric. 2013, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, S.; Al-Absi, K.; Al-Shdiefat, S.; Al-Majali, D.; Hijazean, D. Effect of olive mill wastewater land-spreading on soil properties, olive tree performance and oil quality. Sci. Hortic. 2014, 175, 160–166. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G.; Moutsopoulou, M.; Stefanoudaki, E. Application of olive mill wastewater to a Cretan olive orchard: Effects on soil properties, plant performance and the environment. Agric. Ecosyst. Environ. 2010, 138, 293–298. [Google Scholar] [CrossRef]
- Nektarios, P.A.; Ntoulas, N.; McElroy, S.; Volterrani, M.; Arbis, G. Effect of Olive Mill Compost on Native Soil Characteristics and Tall Fescue Turfgrass Development. Agron. J. 2011, 103, 1524–1531. [Google Scholar] [CrossRef]
- Saadi, I.; Laor, Y.; Raviv, M.; Medina, S. Land spreading of olive mill wastewater: Effects on soil microbial activity and potential phytotoxicity. Chemosphere 2007, 66, 75–83. [Google Scholar] [CrossRef]
- Magdich, S.; Ben Ahmed, C.; Jarboui, R.; Ben Rouina, B.; Boukhris, M.; Ammar, E. Dose and frequency dependent effects of olive mill wastewater treatment on the chemical and microbial properties of soil. Chemosphere 2013, 93, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.; Schiavon, M.; Nardi, S.; Ertani, A.; Celano, G.; Colombo, C.M. Biostimulant Potential of Humic Acids Extracted From an Amendment Obtained via Combination of Olive Mill Wastewaters (OMW) and a Pre-treated Organic Material Derived From Municipal Solid Waste (MSW). Front. Plant Sci. 2018, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Elayadi, F.; El Adlouni, C.; El Herradi, M.A.E.; El Krati, M.; Tahiri, S.; Naman, M.N.F. Effects of raw and treated olive mill wastewater (OMW) by coagulation-flocculation, on the germination and the growth of three plant species (wheat, white beans, lettuce). Moroc. J. Chem. 2019, 7, 111–122. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Müezzinoğlu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Sampedro, I.; Romero, C.; Ocampo, J.A.; Brenes, M.; Garcia, I. Removal of monomeric phenols in dry mill olive residue by saprobic fungi. J. Agri. Food Chem. 2004, 52, 4487. [Google Scholar] [CrossRef]
- García, I.G.; Peña, P.J.; Venceslada, J.B.; Martín, A.M.; Santos, M.M.; Gómez, E.R. Removal of phenol compounds from olive mill wastewater using Phanerochaete chrysosporium, Aspergillus niger, Aspergillus terreus and Geotrichum candidum. Process Biochem. 2000, 35, 751–758. [Google Scholar] [CrossRef]
- Akratos, C.S.; Tekerlekopoulou, A.G.; Vasiliadou, I.A.; Vayenas, D.V. Chapter 8—Cocomposting of olive mill waste for the production of soil amendments. In Galanakis CMBT-OMW; Academic Press: Cambridge, MA, USA, 2017; pp. 161–182. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Current and potential uses of composted olive oil waste. Int. J. Food Sci. Technol. 2007, 42, 281–295. [Google Scholar] [CrossRef]
- Chang, J.I.; Tsai, J.J.; Wu, K.H. Thermophilic composting of food waste. Bioresour. Technol. 2006, 97, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, F.; Chronopoulou, L.; Pizzichini, D.; Lionetti, V.; Fontana, C.; Aromolo, R.; Socciarelli, S.; Gambelli, L.; Bartolacci, B.; Finotti, E.; et al. Olive Mill Wastes: A Source of Bioactive Molecules for Plant Growth and Protection against Pathogens. Biology 2020, 9, 450. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. A study of the recovery of the dietary fibres from olive mill wastewater and the gelling ability of the soluble fibre fraction. LWT 2010, 43, 1009–1017. [Google Scholar] [CrossRef]
- García, M.L.; Cáceres, E.; Selgas, M.D. Utilisation of fruit fibres in conventional and reduced-fat cooked-meat sausages. J. Sci. Food Agric. 2007, 87, 624–631. [Google Scholar] [CrossRef]
- Brozzoli, V.; Bartocci, S.; Terramoccia, S.; Contò, G.; Federici, F.; D’Annibale, A.; Petruccioli, M. Stoned olive pomace fermentation with Pleurotus species and its evaluation as a possible animal feed. Enzym. Microb. Technol. 2010, 46, 223–228. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of agro-industrial by-products, effluents and waste: Concept, opportunities and the case of olive mill wastewaters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Queimada, A.J.; Mota, F. Solubility of multifunctional associating molecules: Measurements and thermodynamic modeling. J. Phys. Chem. B 2009, 113, 3469–3476. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Pereira, R.; Freitas, A.C.; Rocha-Santos, T.A.P.; Panteleitchouk, T.S.L.; Duarte, A.C. Olive oil mill wastewaters before and after treatment: A critical review from the ecotoxicological point of view. Ecotoxicology 2012, 21, 615–629. [Google Scholar] [CrossRef]
- Xue, H.; Xie, W.; Jiang, Z.; Wang, M.; Wang, J.; Zhao, H.; Zhang, X. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016, 46, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of Olive Oil Antioxidants between Oil and Water Phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Takaç, S.; Karakaya, A. Recovery of Phenolic Antioxidants from Olive Mill Wastewater. Recent Pat. Chem. Eng. 2009, 2, 230–237. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Olejniczak, J.; Staniewski, J.; Szymanowski, J. Extraction of phenols and phenyl acetates with diethyl carbonate. Anal. Chim. Acta 2005, 535, 251–257. [Google Scholar] [CrossRef]
- Palma, M.; de Paiva, J.L.; Zilli, M.; Converti, A. Batch phenol removal from methyl isobutyl ketone by liquid–liquid extraction with chemical reaction. Chem. Eng. Process. Process Intensif. 2007, 46, 764–768. [Google Scholar] [CrossRef]
- Obied, H.; Bedgood, D.; Prenzler, P.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Japón-Luján, R.; de Castro, M.D.L. Static–dynamic sequential superheated liquid extraction of phenols and fatty acids from alperujo. Anal. Bioanal. Chem. 2008, 392, 1241–1248. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Klen, T.J.; Vodopivec, B.M. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.-P.; Motilva, M.-J. Development of a Phenol-Enriched Olive Oil with Phenolic Compounds from Olive Cake. J. Agric. Food Chem. 2010, 58, 10396–10403. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Ouazzani, N.; Sayadi, S.; Mandi, L. Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies. J. Hazard. Mater. 2009, 166, 117–125. [Google Scholar] [CrossRef]
- Sabbah, I.; Marsook, T.; Basheer, S. The effect of pretreatment on anaerobic activity of olive mill wastewater using batch and continuous systems. Process Biochem. 2004, 39, 1947–1951. [Google Scholar] [CrossRef]
- Scoma, A.; Bertin, L.; Zanaroli, G.; Fraraccio, S.; Fava, F. A physicochemical–biotechnological approach for an integrated valorization of olive mill wastewater. Bioresour. Technol. 2011, 102, 10273–10279. [Google Scholar] [CrossRef]
- Ferri, F.; Bertin, L.; Scoma, A.; Marchetti, L.; Fava, F. Recovery of low molecular weight phenols through solid-phase extraction. Chem. Eng. J. 2011, 166, 994–1001. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Binary biosorption of phenol and chromium(VI) onto immobilized activated sludge in a packed bed: Prediction of kinetic parameters and breakthrough curves. Sep. Purif. Technol. 2006, 49, 205–216. [Google Scholar] [CrossRef]
- Wu, J.; Yu, H.-Q. Biosorption of phenol and chlorophenols from aqueous solutions by fungal mycelia. Process Biochem. 2006, 41, 44–49. [Google Scholar] [CrossRef]
- Turco, A.; Malitesta, C. Removal of Phenolic Compounds from Olive Mill Wastewater by a Polydimethylsiloxane/oxMWCNTs Porous Nanocomposite. Water 2020, 12, 3471. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Elia, I.; Petalas, A.V.; Halvadakis, C.P. Removal of total phenols from olive-mill wastewater using an agricultural by-product, olive pomace. J. Hazard. Mater. 2008, 160, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Achak, M.; Hafidi, A.; Mandi, L.; Ouazzani, N. Removal of phenolic compounds from olive mill wastewater by adsorption onto wheat bran. Desalination Water Treat. 2014, 52, 2875–2885. [Google Scholar] [CrossRef]
- Senol, A.; Hasdemir, I.; Hasdemir, B.; Kurdaş, I. Adsorptive removal of biophenols from olive mill wastewaters (OMW) by activated carbon: Mass transfer, equilibrium and kinetic studies. Asia-Pacific J. Chem. Eng. 2017, 12, 128–146. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M. Towards a high yield recovery of polyphenols from olive mill wastewater on activated carbon coated with milk proteins: Experimental design and antioxidant activity. Food Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef] [PubMed]
- El-Abbassi, A.; Kiai, H.; Hafidi, A.; García-Payo, M.; Khayet, M. Treatment of olive mill wastewater by membrane distillation using polytetrafluoroethylene membranes. Sep. Purif. Technol. 2012, 98, 55–61. [Google Scholar] [CrossRef]
- Kondo, M.; Sato, H. Treatment of wastewater from phenolic resin process by pervaporation. Desalination 1994, 98, 147–154. [Google Scholar] [CrossRef]
- Hao, X.; Pritzker, M.; Feng, X. Use of pervaporation for the separation of phenol from dilute aqueous solutions. J. Membr. Sci. 2009, 335, 96–102. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.; Adhikari, B. Porous polyurethane urea membranes for pervaporation separation of phenol and chlorophenols from water. Chem. Eng. J. 2008, 138, 215–223. [Google Scholar] [CrossRef]
- Xiao, M.; Zhou, J.; Tan, Y.; Zhang, A.; Xia, Y.; Ji, L. Treatment of highly-concentrated phenol wastewater with an extractive membrane reactor using silicone rubber. Desalination 2006, 195, 281–293. [Google Scholar] [CrossRef]
- Paraskeva, C.; Papadakis, V.; Tsarouchi, E.; Kanellopoulou, D.; Koutsoukos, P. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Comparison of the performance of UF membranes in olive mill wastewaters treatment. Water Res. 2011, 45, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.T.A.; de Freitas, O.M.; Ismael, M.R.C.; Carvalho, J.M. Recovery of phenol from aqueous solutions using liquid membranes with Cyanex 923. J. Membr. Sci. 2007, 305, 313–324. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Khayet, M.; Hafidi, A. Micellar enhanced ultrafiltration process for the treatment of olive mill wastewater. Water Res. 2011, 45, 4522–4530. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Russo, C. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). J. Membr. Sci. 2007, 288, 239–246. [Google Scholar] [CrossRef]
- Borsani, R.; Ferrando, B. Ultrafiltration plant for olive vegetation waters by polymeric membrane batteries. Desalination 1997, 108, 281–286. [Google Scholar] [CrossRef]
- Canepa, P.; Marignetti, N.; Rognoni, U.; Calgari, S. Olive mills wastewater treatment by combined membrane processes. Water Res. 1988, 22, 1491–1494. [Google Scholar] [CrossRef]
- Turano, E.; Curcio, S.; De Paola, M.G.; Calabrò, V.; Iorio, G. An integrated centrifugation–ultrafiltration system in the treatment of olive mill wastewater. J. Membr. Sci. 2002, 209, 519–531. [Google Scholar] [CrossRef]
- Stoller, M.; Chianese, A. Optimization of membrane batch processes by means of the critical flux theory. Desalination 2006, 191, 62–70. [Google Scholar] [CrossRef]
- Stoller, M.; Chianese, A. Technical optimization of a batch olive wash wastewater treatment membrane plant. Desalination 2006, 200, 734–736. [Google Scholar] [CrossRef]
- Stoller, M.; Bravi, M. Critical flux analyses on differently pretreated olive vegetation waste water streams: Some case studies. Desalination 2010, 250, 578–582. [Google Scholar] [CrossRef]
- Akdemir, E.O.; Ozer, A. Investigation of two ultrafiltration membranes for treatment of olive oil mill wastewater. Desalination 2009, 249, 660–666. [Google Scholar] [CrossRef]
- Coskun, T.; Debik, E.; Demir, N.M. Treatment of olive mill wastewaters by nanofiltration and reverse osmosis membranes. Desalination 2010, 259, 65–70. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Olive Biophenols as New Antioxidant Additives in Food and Beverage. ChemistrySelect 2017, 2, 1360–1365. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind. Crop. Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Lavecchia, R.; Zuorro, A. Evaluation of olive pomace as a source of phenolic antioxidants for the production of functional cosmetics. Int. J. Appl. Eng. Res. 2015, 10, 34405–34409. [Google Scholar]
- Pan, B.; Du, W.; Zhang, W.; Zhang, X.; Zhang, Q.; Pan, B.; Lv, L.; Zhang, Q.; Chen, J. Improved Adsorption of 4-Nitrophenol onto a Novel Hyper-Cross-Linked Polymer. Environ. Sci. Technol. 2007, 41, 5057–5062. [Google Scholar] [CrossRef]
- Frascari, D.; Bacca, A.E.M.; Wardenaar, T.; Oertlé, E.; Pinelli, D. Continuous flow adsorption of phenolic compounds from olive mill wastewater with resin XAD16N: Life cycle assessment, cost–benefit analysis and process optimization. J. Chem. Technol. Biotechnol. 2019, 94, 1968–1981. [Google Scholar] [CrossRef]
- Innocenzi, V.; Di Celso, G.M.; Prisciandaro, M. Techno-economic analysis of olive wastewater treatment with a closed water approach by integrated membrane processes and advanced oxidation processes. J. Water Reuse Desalination 2021, 11, 122–135. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Zagklis, D.; Papageorgiou, C.; Paraskeva, C. Technoeconomic Analysis of the Recovery of Phenols from Olive Mill Wastewater through Membrane Filtration and Resin Adsorption/Desorption. Sustainability 2021, 13, 2376. [Google Scholar] [CrossRef]
- Zagklis, D.; A Paraskeva, C. Preliminary design of a phenols purification plant. J. Chem. Technol. Biotechnol. 2019, 95, 373–383. [Google Scholar] [CrossRef]
- Stempfle, S.; Carlucci, D.; de Gennaro, B.C.; Roselli, L.; Giannoccaro, G. Available Pathways for Operationalizing Circular Economy into the Olive Oil Supply Chain: Mapping Evidence from a Scoping Literature Review. Sustainability 2021, 13, 9789. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The circular economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Donner, M.; Radić, I. Innovative Circular Business Models in the Olive Oil Sector for Sustainable Mediterranean Agrifood Systems. Sustainability 2021, 13, 2588. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation (EMF). Towards the Circular Economy. Economic and Business Rationale for an Accelerated Transition. 2013. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/publications/Ellen-MacArthur-Foundation-Towards-the-Circular-Economy-vol.1.pdf (accessed on 11 January 2021).
- Negro, M.J.; Manzanares, P.; Ruiz, E.; Castro, E. The circular economy: The Biorefinery Concept for the Industrial Valorization Results FROM Olive Oil Industry Charis. In Olive Mill Waste; Galanakis, M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 57–78. ISBN 978-0-12-805314-0. [Google Scholar]
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards Circular Economy in the Food System. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Negro, M.J.; Manzanares, P.; Ruiz, E.; Castro, E.; Ballesteros, M. The biorefinery concept for the industrial valorization of residues from olive oil industry. In Olive Mill Waste; Academic Press: New York, NY, USA, 2017; pp. 57–78. [Google Scholar] [CrossRef]
- Ben Harb, M.; Abubshait, S.; Etteyeb, N.; Kamoun, M.; Dhouib, A. Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arab. J. Chem. 2020, 13, 4846–4856. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Fíla, V.; Denis, P.C.; Ruby-Figueroa, R. Current Role of Membrane Technology: From the Treatment of Agro-Industrial by-Products up to the Valorization of Valuable Compounds. Waste Biomass Valorization 2017, 9, 513–529. [Google Scholar] [CrossRef]
- Chebbi, A.; Franzetti, A.; Castro, F.D.; Tovar, F.H.G.; Tazzari, M.; Sbaffoni, S.; Vaccari, M. Potentials of winery and olive oil residues for the production of rhamnolipids and other biosurfactants: A step towards achieving a circular economy model. Waste Biomass Valorization 2021, 12, 4733–4743. [Google Scholar] [CrossRef]
- Cifuentes-Cabezas, M.; Carbonell-Alcaina, C.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Comparison of different ultrafiltration membranes as first step for the recovery of phenolic compounds from olive-oil washing wastewater. Process Saf. Environ. Prot. 2021, 149, 724–734. [Google Scholar] [CrossRef]
- Cristóbal, J.; Caldeira, C.; Corrado, S.; Sala, S. Techno-economic and profitability analysis of food waste biorefineries at European level. Bioresour. Technol. 2018, 259, 244–252. [Google Scholar] [CrossRef]
- Del Pozo, C.; Bartrolí, J.; Puy, N.; Fàbregas, E. Separation of value-added chemical groups from bio-oil of olive mill waste. Ind. Crop. Prod. 2018, 125, 160–167. [Google Scholar] [CrossRef]
- Aguado, R.; Vera, D.; López-García, D.A.; Torreglosa, J.P.; Jurado, F. Techno-Economic Assessment of a Gasification Plant for Distributed Cogeneration in the Agrifood Sector. Appl. Sci. 2021, 11, 660. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Cotana, F.; Nicolini, A.; Coccia, V.; Petrozzi, A.; Formica, A.; Bertini, A. Characterization of Various Biomass Feedstock Suitable for Small-Scale Energy Plants as Preliminary Activity of Biocheaper Project. Sustainability 2020, 12, 6678. [Google Scholar] [CrossRef]
- Costa, M.; Massarotti, N.; Stasi, A.; Cirillo, D.; Villetta, M.L.; Di Blasio, G.; Prati, M.V.; Costagliola, M.A.; Mauro, A.; Vanoli, L.; et al. Innovative plants for distributed poly-generation by residual biomass. In Proceedings of the European Biomass Conference and Exhibition Proceedings, ETA-Florence Renewable Energies, Lisbon, Portugal, 27–30 May 2019; pp. 834–848. [Google Scholar]
- Costa, M.; Buono, A.; Caputo, C.; Carotenuto, A.; Cirillo, D.; Costagliola, M.A.; Di Blasio, G.; La Villetta, M.; Macaluso, A.; Martoriello, G.; et al. The “INNOVARE” Project: Innovative Plants for Distributed Poly-Generation by Residual Biomass. Energies 2020, 13, 4020. [Google Scholar] [CrossRef]
- Maroto, M.D.L.T.; Herrera, J.A.L.C.; Vilar, M.M. Dry olive pomace gasification to obtain electrical energy in a downdraft gasifier. Environ. Eng. 2020, 247, 137–144. [Google Scholar]
- Fragoso, R.; Henriques, A.C.; Gominho, J.; Ochando-Pulido, J.M.; Duarte, E. Integrated management of sewage sludge and olive oil production chain waste: Improving conversion process into biomethane. In Proceedings of the European Biomass Conference and Exhibition Proceedings, ETA-Florence Renewable Energies, Lisbon, Portugal, 27–30 May 2019; pp. 983–986. [Google Scholar]
- Hermoso-Orzáez, M.J.; Mota-Panizio, R.; Carmo-Calado, L.; Brito, P. Gasification of biomass and plastic waste from the disassembly of public lighting luminaires for energy valorization. Case study of circular economy applied to the Alentejo region in Portugal. In Proceedings of the European Biomass Conference and Exhibition Proceedings, Marselle, France, 6–9 July 2020; pp. 422–433. [Google Scholar]
- Hermoso-Orzáez, M.J.; Mota-Panizio, R.; Carmo-Calado, L.; Brito, P. Thermochemical and Economic Analysis for Energy Recovery by the Gasification of WEEE Plastic Waste from the Disassembly of Large-Scale Outdoor Obsolete Luminaires by LEDs in the Alto Alentejo Region (Portugal). Appl. Sci. 2020, 10, 4601. [Google Scholar] [CrossRef]
- Díaz-García, A.; Martínez-García, C.; Cotes-Palomino, T. Properties of Residue from Olive Oil Extraction as a Raw Material for Sustainable Construction Materials. Part I: Physical Properties. Materials 2017, 10, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Font, A.; Soriano, L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. One-part eco-cellular concrete for the precast industry: Functional features and life cycle assessment. J. Clean. Prod. 2020, 269, 122203. [Google Scholar] [CrossRef]
- López-García, A.; Cotes-Palomino, T.; Uceda-Rodríguez, M.; Moreno-Maroto, J.; Cobo-Ceacero, C.; Andreola, N.; Martínez-García, C. Application of Life Cycle Assessment in the Environmental Study of Sustainable Ceramic Bricks Made with ‘alperujo’ (Olive Pomace). Appl. Sci. 2021, 11, 2278. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; Uceda-Rodríguez, M.; Cobo-Ceacero, C.J.; de Hoces, M.C.; Martínlara, M.; Cotes-Palomino, T.; García, A.B.L.; García, C.M. Recycling of ‘alperujo’ (olive pomace) as a key component in the sintering of lightweight aggregates. J. Clean. Prod. 2019, 239, 118041. [Google Scholar] [CrossRef]
- Almeida, R.; Cisneros, F.; Mendes, C.V.; Carvalho, M.G.V.; Rasteiro, M.G.; Gamelas, J.A. Valorisation of invasive plant species in the production of polyelectrolytes. Ind. Crop. Prod. 2021, 167, 113476. [Google Scholar] [CrossRef]
- Bargaoui, M.; Jellali, S.; Azzaz, A.A.; Jeguirim, M.; Akrout, H. Optimization of hybrid treatment of olive mill wastewaters through impregnation onto raw cypress sawdust and electrocoagulation. Environ. Sci. Pollut. Res. 2020, 28, 24470–24485. [Google Scholar] [CrossRef]
- Domingues, E.; Rodrigues, F.; Gomes, J.; Quina, M.; Castro-Silva, S.; Martins, R. Screening of low-cost materials as heterogeneous catalysts for olive mill wastewater Fenton’s peroxidation. Energy Rep. 2020, 6, 161–167. [Google Scholar] [CrossRef]
- Aleandri, M.P.; Chilosi, G.; Muganu, M.; Vettraino, A.; Marinari, S.; Paolocci, M.; Luccioli, E.; Vannini, A. On farm production of compost from nursery green residues and its use to reduce peat for the production of olive pot plants. Sci. Hortic. 2015, 193, 301–307. [Google Scholar] [CrossRef]
- Bechara, E.; Papafilippaki, A.; Doupis, G.; Sofo, A.; Koubouris, G. Nutrient dynamics, soil properties and microbiological aspects in an irrigated olive orchard managed with five different management systems involving soil tillage, cover crops and compost. J. Water Clim. Change 2018, 9, 736–747. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Nogales, R.; Romero, E. Biodegradation of high doses of commercial pesticide products in pilot-scale biobeds using olive-oil agroindustry wastes. J. Environ. Manag. 2017, 204, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Esteves, B.M.; Morales-Torres, S.; Maldonado-Hódar, F.J.; Madeira, L.M. Fitting Biochars and Activated Carbons from Residues of the Olive Oil Industry as Supports of Fe- Catalysts for the Heterogeneous Fenton-Like Treatment of Simulated Olive Mill Wastewater. Nanomaterials 2020, 10, 876. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Chacartegui, R.; Ramirez-Rico, J.; Martinez-Fernandez, J. Performance improvement in olive stone’s combustion from a previous carbonization transformation. Fuel 2018, 228, 254–262. [Google Scholar] [CrossRef]
- Kostas, E.T.; Durán-Jiménez, G.; Shepherd, B.J.; Meredith, W.; Stevens, L.A.; Williams, O.S.A.; Lye, G.J.; Robinson, J.P. Microwave pyrolysis of olive pomace for bio-oil and bio-char production. Chem. Eng. J. 2019, 387, 123404. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative Status and Presence of Bioactive Compounds in Meat from Chickens Fed Polyphenols Extracted from Olive Oil Industry Waste. Sustainability 2017, 9, 1566. [Google Scholar] [CrossRef] [Green Version]
- García-Rodríguez, J.; Ranilla, M.J.; France, J.; Alaiz-Moretón, H.; Carro, M.D.; López, S. Chemical Composition, In Vitro Digestibility and Rumen Fermentation Kinetics of Agro-Industrial By-Products. Animals 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurańska, M.; Banaś, J.; Polaczek, K.; Banaś, M.; Prociak, A.; Kuc, J.; Uram, K.; Lubera, T. Evaluation of application potential of used cooking oils in the synthesis of polyol compounds. J. Environ. Chem. Eng. 2019, 7, 103506. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Angosto, J.M.; Roca, M.J.; Fernández-López, J.A. Removal of Diclofenac in Wastewater Using Biosorption and Advanced Oxidation Techniques: Comparative Results. Water 2020, 12, 3567. [Google Scholar] [CrossRef]
- Vilardi, G.; Ochando-Pulido, J.M.; Verdone, N.; Stoller, M.; Di Palma, L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J. Clean. Prod. 2018, 190, 200–210. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling Agricultural Wastes and By-products in Organic Farming: Biofertilizer Production, Yield Performance and Carbon Footprint Analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef] [Green Version]
- Tallou, A.; Aziz, F.; Garcia, A.J.; Salcedo, F.P.; El Minaoui, F.E.; Amir, S. Bio-fertilizers issued from anaerobic digestion for growing tomatoes under irrigation by treated wastewater: Targeting circular economy concept. Int. J. Environ. Sci. Technol. 2022, 19, 2379–2388. [Google Scholar] [CrossRef]
- Mininni, A.; Fausto, C.; Sofo, A.; Ricciuti, P.; Nuzzo, V.; Xiloyannis, C. How soil microbial biodiversity is modified by soil chemical parameters in differently managed olive orchards. Acta Hortic. 2020, 331–338. [Google Scholar] [CrossRef]
- Attard, J.; McMahon, H.; Doody, P.; Belfrage, J.; Clark, C.; Ugarte, J.A.; Pérez-Camacho, M.N.; Martín, M.D.S.C.; Morales, A.J.G.; Gaffey, J. Mapping and Analysis of Biomass Supply Chains in Andalusia and the Republic of Ireland. Sustainability 2020, 12, 4595. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Gastaldi, M.; Morone, P. A Social Analysis of the Olive Oil Sector: The Role of Family Business. Resources 2019, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Gómez, M.; Zapata, S.; Izquierdo, M.; Jarauta-Córdoba, C.; Annevelink, E.; Snels, J.; Urciuoli, L.; Kougioumtzis, M.A.; Karampinis, E.; Grammelis, P.; et al. From agroindustries to integrated biomass logistics centres. Agroinlog project: Summary of final results. In Proceedings of the European Biomass Conference and Exhibition Proceedings, ETA-Florence Renewable Energies, Online Conference, 6–9 July 2020; pp. 941–952. Available online: https://research.wur.nl/en/publications/from-agroindustries-to-integrated-biomasslogistics-centres-agroi (accessed on 27 August 2021).

















| Factors | OMW I | OMW II | OMW III | OMW IV | OMW V | OMW IV |
|---|---|---|---|---|---|---|
| pH | 6.85 | 5.02 | 4.24 | 5.02 | 4.92 | 5.50 |
| COD (kgm−3) | 9.08 | 44.6 | 20.6 | 134.0 | 135.0 | 23.0 |
| BOD (kgm−3) | 4.75 | Nd | 11.0 | 40.0 | 42.0 | 7.90 |
| PhC * (kgm−3) | 0.03 | 2.54 | 0.61 | 5.40 | 6.16 | 0.25 |
| TS (kgm−3) | 7.30 | 33.1 | 15.1 | 117 | 106 | 18.8 |
| VS (kgm−3) | 7.10 | 28.4 | 9.80 | 94.3 | 79.2 | 12.9 |
| Characteristics | Untreated OMW | Treated OMW |
|---|---|---|
| pH at 25 °C | 5 ± 0.2 | 8.1 ± 0.2 |
| Electrical conductivity (25 °C) (dS m−1) | 8.2 ± 0.1 | 14.2 ± 0.1 |
| Chemical oxygen demand (g L−1) | 53.3 ± 4.8 | 4.5 ± 0.41 |
| Biochemical oxygen demand (g L−1) | 13.42 ± 1.21 | 1.8 ± 0.16 |
| COD/BOD5 | 4 ± 0.72 | 2.5 ± 0.45 |
| Salinity (g L−1) | 6.23 ± 0.56 | 12.1 ± 1.1 |
| Water content (g L−1) | 960.6 ± 19.2 | 984 ± 19.7 |
| Total solids (g L−1) | 39.55 ± 1.98 | 15.9 ± 0.8 |
| Mineral matter (g L−1) | 6.5 ± 0.33 | 10.15 ± 0.51 |
| Volatile solid (g L−1) | 33 ± 1.65 | 4.8 ± 0.24 |
| Total organic carbon (g L−1) | 17.6 ± 0.88 | 3.2 ± 0.16 |
| ortho-diphenols (g L−1) | 8.6 ± 0.86 | 0.77 ± 0.08 |
| Total nitrogen Kjeldhal (g L−1) | 0.5 ± 0.05 | 0.25 ± 0.03 |
| Carbon/Nitrogen | 35.2 ± 7.04 | 12.8 ± 2.56 |
| Toxicity by LUMIStox (% I B) | 99 ± 9 | 30 ± 3 |
| P (mg L−1) | 36 ± 3.6 | 15 ± 1.5 |
| Na (g L−1) | 0.8 ± 0.08 | 0.86 ± 0.09 |
| Cl (g L−1) | 1.45 ± 0.15 | 1.3 ± 0.13 |
| K (g L−1) | 8.6 ± 0.8 | 5.34 ± 0.5 |
| Ca (g L−1) | 0.9 ± 0.09 | 3.2 ± 0.3 |
| Fe (mg L−1) | 23.4 ± 2.3 | 38.3 ± 3.8 |
| Mg (mg L−1) | 186.9 ± 18.7 | 281 ± 28.1 |
| Property | pH | Phenols | COD | TSS | TDS | Ca | Cu | K | Mg | Na | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | - | g/L | g/L | g/L | g/L | mg/L | mg/L | g/L | mg/L | mg/L | µg/L |
| Value | 4.6–5.9 | 0.3–3.0 | 10–50 | 5–21 | 2–35 | 2.1 | 0.9 | 0.2–8 | 1.9 | 0.7 | 33″ |
| R. Time | Raw | Treated Effluent | |||||
|---|---|---|---|---|---|---|---|
| Compound | F1 | F2 | F3 | F1 | F2 | F3 | |
| Benzoic acid | 3.307 | 45.0 | 60 | ND | 0.12 | ND | 5.6 |
| Salicylic acid | 3.518 | 162.5 | 160.9 | 468.5 | 16.9 | 6.4 | 67.4 |
| 2,4 Dihydroxy benzoic acid | 4.300 | 426.5 | 426.8 | 126.0 | ND | ND | ND |
| Tyrosol | 4.849 | 83.8 | 164.7 | 38.4 | ND | ND | ND |
| 2,4 Dyhydroxy bnzaldehyde | 6.38 | 368.7 | 1048 | ND | ND | ND | ND |
| Syringic acid | 7.55 | 119.7 | 610.4 | ND | ND | ND | ND |
| 4-Hydroxy benzoic acid | 9.274 | 426.7 | 120.1 | ND | ND | ND | ND |
| 2,4 Dihydroxy cinnamic acid | 10.499 | ND | 96.1 | ND | ND | ND | ND |
| Syringaldehyde | 13.350 | ND | 354.2 | ND | ND | ND | ND |
| p-Coumaric acid | 17.631 | ND | 466.6 | ND | ND | ND | ND |
| Ferulic acid | 18.087 | ND | 278.5 | ND | ND | ND | ND |
| Oxidation Technique | Conditions | Removal Results | Ref. |
|---|---|---|---|
| Fenton using FeCl3 | Initial conc. of H2O2 5% w/v, [FeCl3]/[H2O2] = 0.1 w/w, Reactor Volume = 3 dm3, agitation speed = 60 rpm, operating time = 180 min, pH = 3, and temperature =298 K | 99.8% of total phenols | [55] |
| AOPs (O3/UV, H2O2/UV) | pH values of 2, 7 and 9 for varying H2O2 dosages between 250 and 1000 mg L−1 at 20 °C | 99% of total phenol | [63,64] |
| Photo-Fenton | Temp. (20 °C), stirring (100 rpm), Addition of Iron sulfate heptahydrate (7.5 mL; 0.5 mol L−1), pH = 4.0, aliquots of H2O2 (30% v/v), UV lamp (VL-6-LC, 245–365 nm) | 100% of phenols | [87] |
| Photo-Fenton | catalysts used: (TiO2-A), (ZrO2) and FAZA, cylindrical photo reactor (0.85 L), UV mercury lamp the UV emitter range from 100 to 280 nm wavelength | 97.44% total phenols | [80] |
| Wet H2O2 photocatalytic oxidation | at 298 K. Irradiation 30 W UV-lamp, 0.5 g L−1 of the catalyst, H2O2 added, 2 × 10−2 M., catalyst: 0.5 g L−1 (Al–Fe)PILC | 86% of caffeic acid and 70% Hydroxytyrosol | [88] |
| Ozonation | ozone concentrations varying from 10 to 70 mg L−1, at ambient temperature, the initial COD 1100 and 44,000 mg L−1 | 80% phenol | [89] |
| Combined Process | Conditions | Purification Achieved | Ref. |
|---|---|---|---|
| Wet H2O2 catalytic oxidation followed by up-flow anaerobic sludge blanket (UASB) reactor | Initial COD = 23,400 mg O2 l−1, Initial TOC = 4250.3 mg L−1, Time of reaction = 90 min, pH 3, Fe2+:H2O2 ratio = 1:10, FeSO4·7H2O = 14 g L−1, H2O2 = 34.3 g L−1, dilution ratio = 1:4 | Reduction in COD, BOD5, TOC and TP by 77%, 78%, 71% and 61%, respectively. | [55] |
| Acid cracking and granular activated carbon adsorption followed by biological process. | optimal contact time (24 h), ptimal GAC dosage (20 g/L), pH value to less than 2, Volumetric Exchange Ratio (0.2), temperature (20 ± 2 °C) | 90% and 76% removal efficiency of COD and TPP, | [90] |
| Photocatalytic and membrane processes | Batch photoreactor containing a catalytic (TiO2) membrane, concentration of H2O2 in the photoreactor was (5 mM), OMW diluted 1:100 v/v, 24 h, high pressure UV lamp (450 W) | Reduction in phenol and COD by 90% and 46–51%, respectively. | [91] |
| Coagulation-flocculation then extraction of phenols, followed solar photo-Fenton. | coagulant: (6.67 g/L FeSO4·7H2O), flocculant: (0.287 g/L FLOCAN 23), extraction for 15 min with ethyl acetate at a solvent to sample ratio of 2:1 (v/v), oxidation for 240 min at 0.2 g/L Fe2+, 5 g/L H2O2 and pH = 3 | 73% of COD removal | [73] |
| Ultrasonic irradiaton combined with biodegradation | sonochemical degradation at power intensity: 7 W/cm2; volume: 700 mL; pH: natural; temperature: 25 ± 1 °C, ultrasound frequency: 351 kHz & 206 kHz. | 80% COD removal efficiency | [18] |
| Fenton and anaerobic biological process | fixed H2O2/COD ratio of 0.20, pH = 3.5 and a H2O2/Fe2+ molar ratio of 15:1, microorganisms immobilized in Sepiolite | 64 to 88% COD reduction, and generation of 281 to 322 cm3 of CH4/g COD removed. | [92] |
| Ozonation and EC processes | Electrocoagulation: 45 mA/cm2 after 70 min by using coupled iron–aluminum electrodes, pH = 6, ultraviolet radiation at 253 nm. | 96.4% COD reduction | [93] |
| Modified surfactant (L167-4S) and a cataionic hydrotropes | Surfactant: sodium polypropylene oxides sulfate (L167-4S), combined with cationic hydrotropes tetra butyl ammonium bromide (TBAB) 1:2 molar ratios. | Phenols recovery achieved was in the range of 99.5–99.8%. | [94] |
| Acid flocculation followed by photocatalytic membrane | Membrane UF, Pretreatment processes Acid fluculation and AF and photo catalysis PC, Jb [9.4 L h−1 m−2], TMPb [9 bar] α [0.0110 L h−2 m−2 bar−1] | Increase the plant productivity by 18–59%. | [20] |
| Combined Ozone/Fenton Process | constant H2O2/Fe2+ molar ratio of 10, ozonation time (60–120 min). | Reduction in color, DOC and BODs by 21%, 49% and 22%, respectively. | [95] |
| Coagulation-flocculation using 0.1 g of γ-Fe2O3 nanoparticles with 1 kg sand | ferric nanoparticles used with sand in a ratio of 0.1 g Fe2O3 and 1 kg of sand, dilution rate for OMW with tap water is (1:0.5), quantities for ferric chloride and lime are (1:0.5) mg L−1 | Reduction in total phenols, BOD5 and COD of 99%, 95.3% and 97.2%, respectively. | [32] |
| Liquid phase extraction, then membrane separation surfactant enhanced aquifer remediation. | solvent (pure water and 50% E-50% W), optimum TPC extracted values were achieved using 40 g of two-phase olive oil and 100 mL solvent, without the addition of HCl, at room temperature 25 °C, after 1 h stirring at 100 rpm. | Reduction in the concentrations of phenols, COD and carbohydrates to 10 mg/L, 284 mg/L and 146 mg/L, respectively. | [96] |
| An integration of applying coagulation method as pre-treatment followed by biological processes. | different levels of pH (4.5, 4.0 and 3.0), constant dosage of two coagulants—aluminum sulfate (Alum) and chitosan equal to 400 mg/L and 100 mg/L, respectively. coagulant dosage, ranging from 400 to 1200 mg/L of alum and 300 to 700 mg/L of chitosan, 60 min of sedimentation. | The removal in the coagulation and biological processes of phenols, COD and TOC were 62.89% and 99.6%; 57.16% and 82.5%; 16.76% and 71.9%, respectively. | [1] |
| Stage 1. “Ozonation, primary sediment separation, Electrocoagulation and lime suspension treatment”. Stage 2. ”Ozonation, oxidation, and adsorption on charcoal”. | Prepared catalyst (O3/(Fe2O3 + CuO)/Clay), pre-ozonated raw OMW has been exposed to electrolytic treatment using a cell supplied with four iron plates as electrodes, lime Ca(OH)2 as suspension was added to adjust the pH to a value of (11–11.5) | Stage 1—59, 86, 70, and 91% of COD, total phenols, color and TS reduction. Stage 2—96% of COD, 100% of total phenols and color and TSS reduction | [97] |
| Coagulation-flocculation, and oxidation with H2O2. | coagulation-flocculation’s optimal conditions are 1.5 g/L of Al2(SO4)3 and 3 g/L of quicklime and a equal to pH = 8.5, hydrogen peroxide at a rate of 30 g/L. | The value of COD removal can reach 91% | [98] |
| Natural flotation followed by anaerobic-aerobic biodegradation | aeration at a rate of 3.5 L/min, Aeration was maintained discontinuously (every 30 min) Phytotoxicity test are conducted in the darkness at room temperature (25 °C) for 120 hr of exposure. | Reduction in turbidity, COD, polyphenol, nitrate, ammonium and phosphorus by 67.5%, 29.1%, 25.2%, 93.9%, 77.1% and 81.8%, respectively. | [99] |
| Infiltration percolation in column followed by aerobic biological treatment | Infiltration using granular activated carbon column mixed with 15% of lime, biological treatment under aerobic conditions. The total volume treated is 1500 mL diluted 15 times with distilled water and neutralized with H2SO4 (0.1 N) | Reduction in COD, BOD5, and polyphenols by 87.86%, 87.39% and 81.59%, respectively. | [100] |
| multi-soil-layering ecotechnology and adsorption on activated carbon/lime | activated carbon adsorbent, (pH = 2, T = 298 K, dilution factor = 5, mass (CA) = 5.5 g) | Reduction in COD, polyphenols and color by 92% 100% and 100%. | [101] |
| Sole and combination of H2O2, O3, and UVA irradiation | Photolysis, ozonation (O3/dark), ozone photolysis (O3/UVA), H2O2 -peroxidation (H2O2/Dark), H2O2, photoperoxidation (H2O2/UVA), peroxonation (H2O2/O3/dark), and photo-peroxonation (H2O2/O3/UVA) | 40% reduction in COD by UVA, increase in BOD5 up to 209% and biodegradability by dark peroxonation of 254% | [102] |
| Microorganism | Treatment Conditions | % Phenolic Reduction | Time | Enzymes |
|---|---|---|---|---|
| Lentinus edodes | “Five-fold diluted OMW in medium containing glucose 5 g/L”. | 65 | 12 d | Phenol oxidases MnP |
| Pleurotus ostreatus | “10% (v/v) water-diluted OMW incubated with fungus previously adapted potato dextrose broth, yeast extract, and maltose medium containing up to 20% OMW” | 90 | 100 h | Phenol oxidases |
| “Abortiporus biennis (CCBAS 521)” | OMW was 50% (v/v) water-diluted, pH was adjusted to 6.0 with H3PO4. The white-rot fungi cultivativation on 50% water diluted OMW plus agar 1.6% (w/v) | 54.5 | Lacc MIP MnP (only observed in P. ostreatus and A. biennis) | |
| “Pleurotus ostreatus (CCBAS 472)” | 51.5 | |||
| “Panellus stipticus (CCBAS 450)” | 42.2 | |||
| “Dichomitus squalens (CCBAS 751)” | 36.4 | 30 d | ||
| “Pleurotus flavido–alba” | “Bioreactor filled with basal medium plus veratryl alcohol (0.43 g/L), Tween 20 (0.5 g/L), and supplemented with Mn (II). After 5 d of fermentation, concentrated and sterilized OMW was added” | 51 | 14 d | MnP Lacc |
| “Pleurotus ostreatus” | Undiluted, thermally processed OMW. | 65 | 21 d | MnP |
| Thermally processed OMW at 100 °C, and water diluted at 50% (v/v). | 67 | 19 d | LiP | |
| 50% (v/v) water-diluted and sterilized, (120 °C, 1 atm) OMW | 78 | 21 d | Lacc | |
| “Pleurotus spp. LGAM P105” | 75% OMW and 25% distilled water, no addition of nutrients or pretreatment | 69 | 24 d | Lacc |
| “Pleurotus spp. LGAM P112” | 71 | |||
| “Pleurotus spp. LGAM P113” | 73 | |||
| “Pleurotus spp. LGAM P116” | 73 |
| Phenolic Compound | Range (mg L−1) | Main Reported Activities |
|---|---|---|
| Hydroxytyrosol | 1073.37–4326.88 | Antioxidant, cardioprotective, Skin bleaching, antiatherogenic, anti-inflammatory, chemo preventive |
| Protocatechuic acid | 66.61–107.31 | antioxidant, antifungal, antihepatotoxic |
| 3, 4-dihydroxyphenylacetic acid | 212.26–253.42 | Antioxidant, hepatoprotective agent |
| Tyrosol | 35.53–920.16 | Antioxidant, anti-inflammatory antiatherogenic, cardioactive |
| Vanillic acid | 8.77–126.64 | antioxidant antimicrobial activity |
| Caffeic acid | 9.59–682.19 | Antioxidant, chemoprotective antiatherogenic, antimicrobial |
| Siringic acid | 1.42–24.00 | antioxidant, antimicrobial, anti-inflammatory |
| p-Cumaric acid | 29.79–341.93 | Antioxidant, antimicrobial chemoprevention |
| Ferulic acid | 23.50–27.12 | antioxidant, anti-inflammatory, |
| Oleuropein | 127.46–5093.49 | Antioxidant, hypoglycemic antihypertensive |
| Luteolin | 84.57–2745.90 | antioxidant, anti-inflammatory, antibacterial, antiviral |
| Mode of Extraction | Solvent | Recovered Phenolic Compounds | % Rec. | Ref. |
|---|---|---|---|---|
| Solid–liquid extraction | MeOH/H2O (80:20, v/v, pH 2), Sodium metabisulfite n-hexane Amberlite XAD7, XAD16, IRA96 and Isolute ENV+, resins | Hydroxytyrosol glucoside; Hydroxytyrosol, Tyrosol; flavonoidal glycoside; caffeic acid; verbascoside; rutin; verbascoside isomer; oleuropein derivative; oleuropein; oleuropein isomer; luteolin. Hydroxytyrosol and others | 84 | [138,151] |
| Liquid-Liquid or Hydrolysis Liquid-solid extraction From waste cake | Ultrasonic probe and MeOH. Ethanol, a mixture of ethanol to water of 1:1 n-propanol MeOH NaOH (pH 12) HCl (pH 2) Ethyl acetate | Hydroxytyrosol and glucoside form; Tyrosol; verbascoside; verbascoside derivative; luteolin-7-O-glucoside; rutin; p-HPEA-EDA; comselogoside Hydroxytyrosol, oleuropein, luteolin, hesperidin, catechin, cyanidin glycosides and caffeic, ferulic, chlorogenic, pyrocatechinic, syringic, o-coumaric, p-coumaric, cinnamic and trans-cinnamic acids Gallic, protocatechuic; hydroxybenzoic, vanillic; caffeic; sinapic, rutin, p-coumaric, hesperidin, quercetin and cinnamic acids | 95.3 92.2 90.7 30 90 | [138,151] |
| Ultrasound-assisted extraction (UAE) | n-hexane ethyl acetate. EtOH/H2O (80:20, v/v). | Tyrosol; Hydroxytyrosol; Hydroxytyrosol acetate; luteolin; vanillin; vanillic acid; p-coumaric acid; caffeic acid; 3,4-DHPEA-EDA; 3,4-; DHPEA-EA; methyl 3,4-DHPEA-EA oleuropein and many derivatives | Not given | [138,151] |
| Pressurized liquid extraction | MeOH/H2O (80:20, v/v). | Hydroxytyrosol; verbascoside; luteolin-7-O-glucoside; apigenin-7-O-glucoside; oleuropein; luteolin; apigenin, diosmetin. | Not given | [138,151] |
| Adsorbent | qmax | KL | References |
|---|---|---|---|
| Olive pomace | 11.4 | 0.005 | [204] |
| Banana peel | 688.9 | 0.24 | [197] |
| Wheat bran | 478.3 | 0.13 | [205] |
| Activated carbon | 268.17 | 0.14 | [206] |
| Activated carbon coated with milk protein | 246.45 | 9.1 | [207] |
| PDMS/oxMWCNTs | 454.55 | 0.014 | [203] |
| Type of Membrane Process | Main Results | Ref. |
|---|---|---|
| Direct contact membrane distillation using polytetrafluoroethylene membranes | 100% phenol separation | [208] |
| UF with regenerated cellulose membranes/NF/RO | 0.5 and 30 g/L total polyphenols concentrated | [214] |
| Liquid membrane with 2% Cyanex 923 | 90–97% phenol was rejected | [215] |
| Micellar enhanced UF/anionic surfactant (sodium dodecyl sulfate salt, SDS)/hydrophobic polyvinylidene fluoride membrane | 74% polyphenols were rejected | [216] |
| NF/MF/osmoticdistillation/vacuum membrane distillation | Recovery of 78% of the initial content of polyphenols | [217] |
| MF/UF/RO consists of a polymeric hydraulics membrane | Retentate containing 464.870 mg/L with free low MW polyphenols | [218] |
| Oil removal and TSS settling/UF/permeate mix with sewage and double-stage biological treatment | 50% COD load decrease after UF and up to 70% after biological treatment | [219] |
| UF/treatment with adsorbing polymers and RO | COD reduction (UF) up to 63%, 93% (RO) and 99% total | [220] |
| Centrifugation and UF of centrifuge supernatant | 55% COD, 80% ashes, and TSS reductions after centrifugation, 90% final COD abatement | [221] |
| pretreatment among flocculation/UV-TiO2 photocatalysis/aerobic digestion/MF, followed by UF + NF + RO | Overall COD abatement 98.8–99.4% | [222,223,224] |
| pH adjustment/cartridge filtration and UF | COD, TOC and SS removal ratios 92.3%, 92.7%, and 97.1% | [225] |
| Centrifugation/UF/NF and RO | COD removal 59.4–79.2% for NF, whereas 96.2–96.3% for RO | [226] |
| MF, NF, and OD or VMD | MF, 91% and 26% for TSS and TOC reduction, respectively. NF removed 63% TOC, and TC reduction in MF permeate | [217] |
| ID | Name of the Pathway | No. of Total Studies | Some Studies Related to Each Pathway |
|---|---|---|---|
| #1 | High-added value bioactive compounds recovery from olive mill waste, olive leaves or waste cooking oil | 24 | [245,246,247,248,249,250,251,252] |
| #2 | Biofuel production from pruning residues and/or olive mill wastes, or waste cooking oil | 23 | [252,253,254,255,256,257,258,259] |
| #3 | Olive mill waste reused as component in the manufacture of sustainable building materials | 8 | [260,261,262,263] |
| #4 | Olive mill wastewater reused for soil conditioning/fertilization/irrigation | 6 | [264,265,266] |
| #5 | Pruning residues and/or olive mill waste valorized for regenerative agriculture | 6 | [267,268,269] |
| #6 | Biochar (bio-oil, syngas) production from olive mill waste and/or pruning residues | 6 | [270,271,272] |
| #7 | Olive leaves or olive cake reused for animal feed | 4 | [273,274] |
| #8 | Polymeric biomaterials production from pruning residues, olive mill by-products or waste cooking oil | 4 | [275,276] |
| #9 | Olive mill waste recycled as bio-adsorbent material for treating aqueous effluents | 3 | [277,278] |
| #10 | Biofertilizers or bio-stimulants and biopesticides production from olive mill waste | 3 | [279] |
| #11 | Treated urban/industrial wastewater reused for agricultural purposes | 2 | [280] |
| n.a | Miscellanea: collection of studies that are not focused on a specific pathway | 12 | [281,282,283,284] |
| Process | The Conventional OMW Treatment Process | Sustainable OMW Treatment Process | |
|---|---|---|---|
| Parameter | |||
| Sustainability | Less sustainable | More sustainable | |
| Environmental effect | Less friendly | More friendly | |
| Recovery of valuable compounds | None to low | High | |
| Energy consumption | High | Yes because of the possible use of renewable energy (Solar energy) | |
| Products economic value | low | High due to the valuable products | |
| Production of waste Materials | high due to different types of solid wastes produced | Low | |
| Use of new separation technologies | Rare | Very necessary | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qodah, Z.; Al-Zoubi, H.; Hudaib, B.; Omar, W.; Soleimani, M.; Abu-Romman, S.; Frontistis, Z. Sustainable vs. Conventional Approach for Olive Oil Wastewater Management: A Review of the State of the Art. Water 2022, 14, 1695. https://doi.org/10.3390/w14111695
Al-Qodah Z, Al-Zoubi H, Hudaib B, Omar W, Soleimani M, Abu-Romman S, Frontistis Z. Sustainable vs. Conventional Approach for Olive Oil Wastewater Management: A Review of the State of the Art. Water. 2022; 14(11):1695. https://doi.org/10.3390/w14111695
Chicago/Turabian StyleAl-Qodah, Zakaria, Habis Al-Zoubi, Banan Hudaib, Waid Omar, Maede Soleimani, Saeid Abu-Romman, and Zacharias Frontistis. 2022. "Sustainable vs. Conventional Approach for Olive Oil Wastewater Management: A Review of the State of the Art" Water 14, no. 11: 1695. https://doi.org/10.3390/w14111695