Transcriptomic Analysis of the Molecular Response Mechanism of Microcystis aeruginosa to Iron Limitation Stress
Abstract
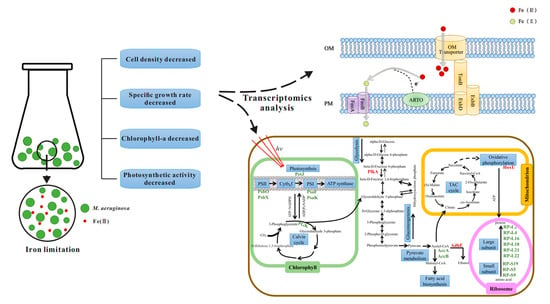
:1. Introduction
2. Materials and Methods
2.1. Culture of Microcystis aeruginosa
2.2. Experimental Design
2.3. Physiological Index Measurement
2.3.1. Cell Density
2.3.2. Determination of Chlorophyll-a and Carotenoid Content
2.3.3. Determination of Chlorophyll Fluorescence
2.4. RNA Extraction and Determination
2.5. Genome Annotation and Analysis
3. Results and Discussion
3.1. Effect of Iron Limitation on Microcystis aeruginosa Growth
3.2. Effects of Iron Limitation on Genes Related to Iron Absorption and Transport
3.3. The Effect of Iron Limitation on Photosynthesis
3.4. Effect of Iron Limitation on the Molecular Regulatory Network of Microcystis aeruginosa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.P.; Häder, D.-P.; Sinha, R.P. Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res. Rev. 2010, 9, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.C.; Bourassa, J.S.; Masse, E. Cellular Homeostasis: A Small RNA at the Crossroads of Iron and Photosynthesis. Curr. Biol. 2017, 27, R380–R383. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Ameijeiras, S.; Cosio, C.; Hassler, C.S. Long-Term Acclimation to Iron Limitation Reveals New Insights in Metabolism Regulation of Synechococcus sp. PCC7002. Front. Mar. Sci. 2017, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Larson, C.A.; Mirza, B.; Rodrigues, J.L.M.; Passy, S.I. Iron limitation effects on nitrogen-fixing organisms with possible implications for cyanobacterial blooms. FEMS Microbiol. Ecol. 2018, 94, 1–8. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Milligan, A.J. Photophysiological expressions of iron stress in phytoplankton. Annu. Rev. Mar. Sci. 2013, 5, 217–246. [Google Scholar] [CrossRef]
- North, R.L.; Guildford, S.J.; Smith, R.E.H. Evidence for phosphorus, nitrogen, and iron colimitation of phytoplankton communities in Lake Erie. Limnol. Oceanogr. 2007, 52, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Shaked, Y.; Erel, Y.; Sukenik, A. The biogeochemical cycle of iron and associated elements in Lake Kinneret. Geochim. Cosmochim. Acta 2004, 68, 1439–1451. [Google Scholar] [CrossRef]
- Teutsch, N.; Schmid, M.; Müller, B.; Halliday, A.N.; Bürgmann, H.; Wehrli, B. Large iron isotope fractionation at the oxic–anoxic boundary in Lake Nyos. Earth Planet. Sci. Lett. 2009, 285, 52–60. [Google Scholar] [CrossRef]
- Kranzler, C.; Lis, H.; Shaked, Y.; Keren, N. The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ. Microbiol. 2011, 13, 2990–2999. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.-W.; Lou, W.-J.; Sun, C.-Y.; Yang, N.; Li, Z.-K.; Li, D.-L.; Zang, S.-S.; Fu, F.-X.; Hutchins, D.A.; Jiang, H.-B.; et al. Outer Membrane Iron Uptake Pathways in the Model Cyanobacterium Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2018, 84, e01512-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.-B.; Lou, W.-J.; Ke, W.-T.; Song, W.-Y.; Price, N.M.; Qiu, B.-S. New insights into iron acquisition by cyanobacteria: An essential role for ExbB-ExbD complex in inorganic iron uptake. ISME J. 2015, 9, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Fujii, M.; Dang, T.C.; Rose, A.L.; Omura, T.; Waite, T.D. Effect of light on iron uptake by the freshwater cyanobacterium Microcystis aeruginosa. Environ. Sci. Technol. 2011, 45, 1391–1398. [Google Scholar] [CrossRef]
- Xing, W.; Huang, W.M.; Li, D.H.; Liu, Y.D. Effects of iron on growth, pigment content, photosystem II efficiency, and siderophores production of Microcystis aeruginosa and Microcystis wesenbergii. Curr. Microbiol. 2007, 55, 94–98. [Google Scholar] [CrossRef]
- Liu, S.-W.; Qiu, B.-S. Different responses of photosynthesis and flow cytometric signals to iron limitation and nitrogen source in coastal and oceanic Synechococcus strains (Cyanophyceae). Mar. Biol. 2012, 159, 519–532. [Google Scholar] [CrossRef]
- Wan, M.; Jin, X.; Xia, J.; Rosenberg, J.N.; Yu, G.; Nie, Z.; Oyler, G.A.; Betenbaugh, M.J. The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2014, 98, 9473–9481. [Google Scholar] [CrossRef]
- Makower, A.K.; Schuurmans, J.M.; Groth, D.; Zilliges, Y.; Matthijs, H.C.P.; Dittmann, E. Transcriptomics-Aided Dissection of the Intracellular and Extracellular Roles of Microcystin in Microcystis aeruginosa PCC 7806. Appl. Environ. Microbiol. 2015, 81, 544–554. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.T.; Sinclair, G.A.; Wawrik, B. Transcriptome analysis of Scrippsiella trochoidea CCMP 3099 reveals physiological changes related to nitrate depletion. Front. Microbiol. 2016, 7, 639. [Google Scholar] [CrossRef] [Green Version]
- Bolton, J.J. Algal culturing techniques. J. Exp. Mar. Biol. Ecol. 2006, 336, 262. [Google Scholar] [CrossRef]
- Fujii, M.; Dang, T.C.; Bligh, M.W.; Waite, T.D. Cellular characteristics and growth behavior of iron-limited Microcystis aeruginosa in nutrient-depleted and nutrient-replete chemostat systems. Limnol. Oceanogr. 2016, 61, 2151–2164. [Google Scholar] [CrossRef]
- Fu, Q.-L.; Fujii, M.; Natsuike, M.; Waite, T.D. Iron uptake by bloom-forming freshwater cyanobacterium Microcystis aeruginosa in natural and effluent waters. Environ. Pollut. 2019, 247, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, H.; Juneau, P.; Qiu, B. Comparative studies on the photosynthetic responses of three freshwater phytoplankton species to temperature and light regimes. J. Appl. Phycol. 2012, 24, 1113–1122. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; Kulk, G.; Buma, A.G.J.; Visser, R.J.W.; Van Dijken, G.L.; Mills, M.M.; Arrigo, K.R. The effect of iron limitation on the photophysiology of phaeocystis antarctica (prymnesiophyceae) and fragilariopsis cylindrus (bacillariophyceae) under dynamic irradiance1. J. Phycol. 2012, 48, 45–59. [Google Scholar] [CrossRef]
- Fujii, M.; Rose, A.L.; Omura, T.; Waite, T.D. Effect of Fe(II) and Fe(III) Transformation Kinetics on Iron Acquisition by a Toxic Strain of Microcystis aeruginosa. Environ. Sci. Technol. 2010, 44, 1980–1986. [Google Scholar] [CrossRef]
- Goto, T.; Aoki, R.; Minamizaki, K.; Fujita, Y. Functional Differentiation of Two Analogous Coproporphyrinogen III Oxidases for Heme and Chlorophyll Biosynthesis Pathways in the Cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2010, 51, 650–663. [Google Scholar] [CrossRef] [Green Version]
- Masoumi, A.; Heinemann, I.U.; Rohde, M.; Koch, M.; Jahn, M.; Jahn, D. Complex formation between protoporphyrinogen IX oxidase and ferrochelatase during haem biosynthesis in Thermosynechococcus elongatus. Microbiology 2008, 154, 3707–3714. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.C. The Ferritin-like superfamily: Evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1800, 691–705. [Google Scholar] [CrossRef]
- Kaushik, M.S.; Srivastava, M.; Mishra, A.K. Iron Homeostasis in Cyanobacteria, Cyanobacteria; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 245–260. [Google Scholar]
- Katoh, H.; Hagino, N.; Ogawa, T. Iron-binding activity of FutA1 subunit of an ABC-type iron transporter in the cyanobacterium Synechocystis sp. Strain PCC 6803. Plant Cell Physiol. 2001, 42, 823–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranzler, C.; Lis, H.; Finkel, O.M.; Schmetterer, G.; Shaked, Y.; Keren, N. Coordinated transporter activity shapes high-affinity iron acquisition in cyanobacteria. ISME J. 2014, 8, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lee, H.; Shin, D. The FeoA protein is necessary for the FeoB transporter to import ferrous iron. Biochem. Biophys. Res. Commun. 2012, 423, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Perri, K.A.; Manning, S.R.; Watson, S.B.; Fowler, N.L.; Boyer, G.L. Dark adaptation and ability of pulse-amplitude modulated (PAM) fluorometry to identify nutrient limitation in the bloom-forming cyanobacterium, Microcystis aeruginosa (Kutzing). J. Photochem. Photobiol. B-Biol. 2021, 219, 9. [Google Scholar] [CrossRef]
- Juneau, P.; Green, B.R.; Harrison, P.J. Simulation of Pulse-Amplitude-Modulated (PAM) fluorescence: Limitations of some PAM-parameters in studying environmental stress effects. Photosynthetica 2005, 43, 75–83. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, L. Research progress in chloroplast iron transport proteins. Plant Physiol. J. 2017, 53, 9–16. [Google Scholar] [CrossRef]
- Diaz-Quintana, A.; Navarro, J.A.; Hervas, M.; Molina-Heredia, F.P.; De la Cerda, B.; De la Rosa, M.A. A comparative structural and functional analysis of cyanobacterial plastocyanin and cytochrome c (6) as alternative electron donors to Photosystem I. Photosynth. Res. 2003, 75, 97–110. [Google Scholar] [CrossRef]
- Navarro, J.A.; Durán, R.V.; Miguel, A.; Hervás, M. Respiratory cytochrome c oxidase can be efficiently reduced by the photosynthetic redox proteins cytochrome c6 and plastocyanin in cyanobacteria. FEBS Lett. 2005, 579, 3565–3568. [Google Scholar] [CrossRef]
- Dibrova, D.V.; Shalaeva, D.N.; Galperin, M.Y.; Mulkidjanian, A.Y. Emergence of cytochrome bc complexes in the context of photosynthesis. Physiol. Plant. 2017, 161, 150–170. [Google Scholar] [CrossRef] [Green Version]
- Peers, G.; Price, N.M. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef]
- Nodop, A.; Pietsch, D.; Hocker, R.; Becker, A.; Pistorius, E.K.; Forchhammer, K.; Michel, K.P. Transcript profiling reveals new insights into the acclimation of the mesophilic fresh-water cyanobacterium Synechococcus elongatus PCC 7942 to iron starvation. Plant Physiol. 2008, 147, 747–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, J.; Steglich, C.; Scholz, I.; Hess, W.R.; Kirilovsky, D. Inverse regulation of light harvesting and photoprotection is mediated by a 3′-end-derived sRNA in cyanobacteria. Plant Cell 2021, 33, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Blankenship, R.E. Both forward and reverse TCA cycles operate in green sulfur bacteria. J. Biol. Chem. 2010, 285, 35848–35854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Hakamada, T.; Ogino, T.; Osanai, T. Reconstitution of oxaloacetate metabolism in the tricarboxylic acid cycle in Synechocystis sp. PCC 6803: Discovery of important factors that directly affect the conversion of oxaloacetate. Plant J. 2021, 105, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Figge, R.M.; Cassier-Chauvat, C.; Chauvat, F.; Cerff, R. The carbon metabolism-controlled Synechocystis gap2 gene harbours a conserved enhancer element and a Gram-positive-like-16 promoter box retained in some chloroplast genes. Mol. Microbiol. 2000, 36, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.K.; Fu, H.X.; Ren, M.M.; Liao, Z.P.; Ma, Y.; Wang, J.F. Enhanced butyric acid production in Clostridium tyrobutyricum by overexpression of rate-limiting enzymes in the Embden-Meyerhof-Parnas pathway. J. Biotechnol. 2018, 272, 14–21. [Google Scholar] [CrossRef]
- Thelen, J.J.; Ohlrogge, J.B. Metabolic Engineering of Fatty Acid Biosynthesis in Plants. Metab. Eng. 2002, 4, 12–21. [Google Scholar] [CrossRef]
- Foster, D.W. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J. Clin. Investig. 2012, 122, 1958–1959. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Wu, G.G.; Shao, W.L. The aldehyde/alcohol dehydrogenase (AdhE) in relation to the ethanol formation in Thermoanaerobacter ethanolicus JW200. Anaerobe 2008, 14, 125–127. [Google Scholar] [CrossRef]
- Pei, J.; Zhou, Q.; Jiang, Y.; Le, Y.; Li, H.; Shao, W.; Wiegel, J. Thermoanaerobacter spp. control ethanol pathway via transcriptional regulation and versatility of key enzymes. Metab. Eng. 2010, 12, 420–428. [Google Scholar] [CrossRef]
- Loveland, A.B.; Korostelev, A.A. Structural dynamics of protein S1 on the 70S ribosome visualized by ensemble cryo-EM. Methods 2018, 137, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, J.; Du, Z.; Shu, Q.; Zheng, Z.; Luo, X. Transcriptomic Analysis of the Molecular Response Mechanism of Microcystis aeruginosa to Iron Limitation Stress. Water 2022, 14, 1679. https://doi.org/10.3390/w14111679
Chen X, Wang J, Du Z, Shu Q, Zheng Z, Luo X. Transcriptomic Analysis of the Molecular Response Mechanism of Microcystis aeruginosa to Iron Limitation Stress. Water. 2022; 14(11):1679. https://doi.org/10.3390/w14111679
Chicago/Turabian StyleChen, Xiaxia, Jie Wang, Zunqing Du, Qihang Shu, Zheng Zheng, and Xingzhang Luo. 2022. "Transcriptomic Analysis of the Molecular Response Mechanism of Microcystis aeruginosa to Iron Limitation Stress" Water 14, no. 11: 1679. https://doi.org/10.3390/w14111679