EASD 2019 (European Association for the Study of Diabetes)
September 16-20, 2019; Barcelona, Spain; Day #3 Highlights – Draft
Executive Highlights
-
EASD 2019 keeps moving, with Day #3 complete and brimming with loads of new data.
-
Full results from the VERIFY trial were presented, demonstrating important benefits of early combo therapy of Novartis’ DPP-4 inhibitor Galvus (vildagliptin) and metformin vs. sequential intensification. Treatment with combination therapy resulted in a significant 26-month delay in the primary outcome of treatment failure (defined as reaching A1C > 7%) and treatment intensification compared to monotherapy (p<0.0001). If this is not a siren call to start therapy when diabetes is diagnosed, we don’t know what could be.
-
We also got an early look at full results of the CONCLUDE trial pitting Novo Nordisk’s Tresiba (insulin degludec) vs. Sanofi’s Toujeo (insulin glargine U300), showing no significant difference between the two highly efficacious next-gen basals on the primary endpoint of overall hypoglycemia. For the primary endpoint, rates of overall symptomatic hypoglycemia did not reach significance (RR=0.88; 95% CI: 0.73-1.06; p=0.17). Sanofi’s BRIGHT trial of Toujeo vs. Tresiba found overall similar A1c reductions with the two agents, but a predefined subgroup analysis (first presented at ADA 2019 and also at a poster here at EASD 2019) found significantly different mean A1c changes favoring Toujeo at lower baseline eGFR (p-value for interaction=0.02). On hypoglycemia, BRIGHT found a statistically significant decrease in incidence of hypoglycemia <70 mg/dl (OR=0.74, 95% CI: 0.57-0.97, p=0.03) and hypoglycemia <54 mg/dl (OR=0.63, 95% CI: 0.40-0.99, p=0.044) with Toujeo vs. Tresiba during the first 12 weeks of the study – the “titration period.” Big picture, the message is all about individualization – getting everyone on basal on a next-generation insulin – and then, making sure eGFRs are being measured (this is getting easier) and then prescribing based on whether that sub-group needs something specific.
-
We heard a bold call to action from Dr. Itamar Raz, who advocated for use of SGLT-2s in every person with diabetes with an A1c above 6.5%. As part of a session on results and implications of DECLARE (Farxiga’s CVOT), Dr. Raz implored the community to adopt these agents earlier in treatment. In her independent commentary, the very highly regarded Dr. Melanie Davis emphasized the importance of targeting patients who may benefit most from treatment at first.
-
CGM ruled the day in tech, including the DCCT/EDIC cohort wearing FreeStyle Libre Pro (just 9% met the >70% time-in-range goal), full three-year data from COMISAIR, and a GOLD post-hoc analysis. All three studies continued to build the evidence supporting CGM and time-in-range outcomes, with the emphasis that there remains a long road to get this technology to everyone with type 1 diabetes and to achieve optimal outcomes with it. We’d like to get it to everyone (real-time or intermittent) with diabetes as soon as possible, and to make sure that the ROI is very, very high.
-
We also have a first round of technology exhibit hall coverage: a look at Novo Nordisk’s Bluetooth-enabled disposable insulin pen attachment (prototype), Abbott’s NovoPen 6 + FreeStyle Libre featured display, Medtronic’s new Envision Pro CGM, and and the CE-Marked GlucoMen Day CGM “powered by Waveform.” As a reminder, the heavy CGM + smart pen marketing came alongside the two major tech announcements of the week: Abbott & Sanofi and Medtronic & Novo Nordisk.
-
As promised, we’re also back with some of our favorite quotes and commentary from The diaTribe Foundation’s 6th Annual Solvable Problems in Diabetes event, which featured Profs Stephanie Amiel, Juliana Chen, Chantal Mathieu, and Tina Vilsbøll joining Kelly Close in conversation about the field. The speakers showed off their incredible range of diabetes expertise, touching on what they’re most looking forward to at EASD 2019, individualization of care and improving population level outcomes, new ESC guidelines, diabetes advocacy, and much more. Check out some of our favorite quotes from the night below and stay tuned for broader coverage.
Hola from Barcelona! Day #3 of EASD packed a bunch, with numerous important data readouts happening. See below for our deep dive, and if you missed it, see our highlights reports from Day #1 and Day #2 as well.
Table of Contents []
-
Diabetes Therapy Highlights
- 1. VERIFY Full Results: Early Combo Therapy of DPP-4 Galvus + Metformin Superior to Monotherapy and Sequential Intensification; Doubles Time to Treatment Failure, Provides Added Glycemic Durability, and Shows Intriguing Potential CV Risk Reduction
- 2. CONCLUDE Trial of Tresiba vs. Toujeo in Type 2 Finds No Significant Difference on Primary Endpoint of Overall Symptomatic Hypoglycemia; Exploratory Secondary Endpoints of Nocturnal Hypoglycemia and Severe Hypoglycemia Benefit Trend Toward Tresiba
- 3. Dr. Itamar Raz’s Call to Action: “We Should Be Using SGLT-2s in Every Patient w/ A1c >6.5%” – Conversely, Dr. Melanie Davies Urges More Careful Use in Patients Who Can Benefit Most
- 4. DEFINE-HF: Farxiga Improves KCCQ But Not Primary Endpoint of NTproBNP
- 5. SGLTs in Type 1: Are We Being Overcautious + Not Considering Potential CV and Renal Benefits? Impassioned Calls for a Type 1 CVOT Grow!
- 6. Additional Commentary on Positive Renal Data from DECLARE: Consistent Effects in Patients with Relatively Normal Kidney Function Emphasized
- 7. Ellipse Trial Shows Encouraging Results for Liraglutide in Adolescents with Type 2 Diabetes
- 8. A Role for GLP-1 Agonists and GLP-1/GIP Dual Agonists in Treating Alzheimer’s and Parkinson’s?
-
Diabetes Technology Highlights
- 1. DCCT/EDIC Cohort Wearing FreeStyle Libre Pro: Only 9% Achieved >70% TIR Target, Up to 19% in Those With Automatic Glucose Suspend
- 2. COMISAIR Full Three-Year Data (n=94): CGM Drives Improved Glycemic Outcomes Regardless of Insulin Delivery Method in Type 1s; Fewer Hypoglycemia Episodes and Five Increased Time-in-Range
- 3. GOLD Post-Hoc Analysis: CGM Delivers Benefits Regardless of SMBG Frequency; Jaeb’s Kellee Miller Recaps WISDM Trial
- 4. NHS Data shows A1c Reduction of 0.6% on Pump Initiation (n=505 Type 1s) After Five Years, ~2% Reduction for Baseline A1c ≥10%
- 5. Severe Hypo Elevates Risk of Future Mortality in Type 1 Welsh Cohort from 2000-2018
- Exhibit Hall
- The diaTribe Foundation’s Sixth Annual Solvable Problems in Diabetes Forum
Diabetes Therapy Highlights
1. VERIFY Full Results: Early Combo Therapy of DPP-4 Galvus + Metformin Superior to Monotherapy and Sequential Intensification; Doubles Time to Treatment Failure, Provides Added Glycemic Durability, and Shows Intriguing Potential CV Risk Reduction
Very positive full results from VERIFY (n=2,001) revealed that early combination therapy of metformin and Novartis’ DDP-4 inhibitor Galvus (vildagliptin) doubled the time to initial treatment failure vs. metformin monotherapy. Full results were also published in The Lancet (see here). Treatment with combination therapy resulted in a significant 26-month delay in the primary outcome of treatment failure (defined as reaching A1C > 7%) and treatment intensification compared to monotherapy (p<0.0001). Specifically, those on initial monotherapy received surpassed an A1c of 7% at a median 36 months, compared to 62 months for those on early combination (vildagliptin + metformin). Treatment with combination therapy also resulted in significantly delayed insulin therapy (p<0.0001), meeting the secondary outcome in favor of combination therapy. Results from VERIFY are highly notable, as they present strong evidence for the first time regarding benefits of early combination therapy on durable glycemic control. Moreover, exploratory analysis of the trial also demonstrated intriguing cardiovascular outcomes – in a small sample, but hypothesis generating nonetheless (see below for more). KOL reaction was positive toward VERIFY, and Dr. Dan Drucker summed up implications from the trial quite well in his Twitter musings: “What does VERIFY teach us about treating type 2 diabetes? Diabetes is a serious disease! Think more like an oncologist (early combination therapy) rather than a traditional treat to failure approach. Well tolerated drug combinations with established safety profiles should be used earlier.” This is a brilliant approach – many are already viewing VERIFY as an important evidence base for tackling therapeutic inertia through early combo therapy use. Moreover, with DPP-4 inhibitors soon becoming generic, we see immense possible for accessible and affordable combination therapies of DPP-4s + metformin. And, investments should be made now. For historical context, see a talk by Dr. John Buse what we were writing about DPP-4 inhibitors back in 2007.
-
VERIFY Introduction and Research Question: Dr. Chantal Mathieu started the session off with an overview of the premise of the VERIFY trial, which aims to answer the question of whether providers should initiate combination therapy at the start of glucose-lowering treatment in patients with type 2 diabetes. This overarching theme distills into three clinical questions: (i) Do those with type 2 diabetes benefit from combined therapy at the beginning of pharmacological treatment; (ii) Do they benefit more from combination therapy versus additive therapy; and (iii) Does it matter clinically? Early combination therapy has potential to synergistically target many of diabetes’ pathophysiological mechanisms and lead to better outcomes by preventing or slowing beta-cell function decline without increasing the risk of adverse events or comorbidities. There’s currently little evidence on whether coupling medications as first line therapy creates an additive or protective effect in health outcomes.
-
Background, Design, and Outcomes: Dr. Michael Stumvoll explained that starting combination therapy early may provide an opportunity for reaching glycemic targets before A1c rises to hard to manage levels. Very notably, treatment in VERIFY, a randomized, double-blind, two arm, parallel-group trial that spanned five years, starts at the earliest time and the lowest A1c of all other large-scale anti-hyperglycemic drug efficacy trials (!). All enrolled patients were between 18 and 70 years old, drug-naïve, recently diagnosed with type 2 diabetes (≤24 months), and had eGFRs ≥60 mL/min, BMIs between 22 and 40 kg/m2, and A1cs between 6.5% and 7.5%.
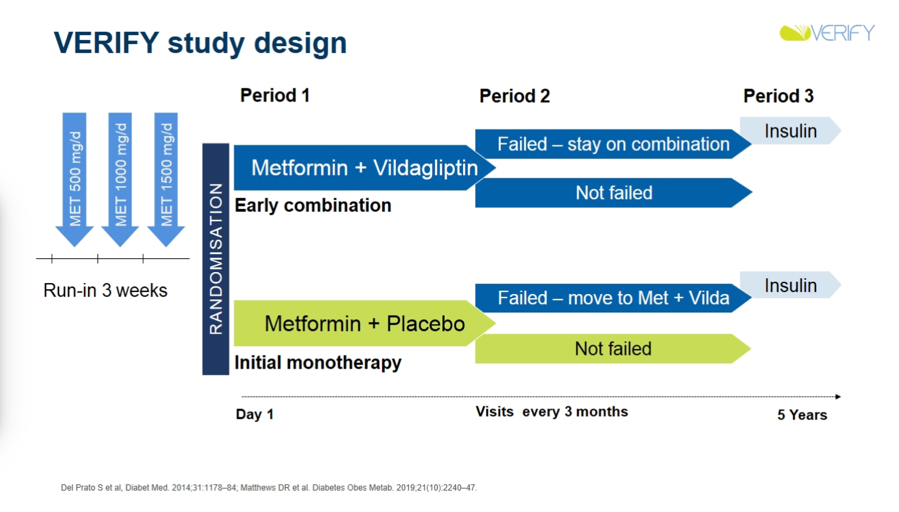
-
VERIFY’s unique trial design featured three periods, with two transition points aligned with the primary and secondary outcomes. Patients were randomized to the early combination therapy group (metformin and Vildagliptin) or monotherapy group (placebo and metformin) in “period one.” If a patient’s A1c surpassed 7%, they either stayed on combination therapy (if started on combination therapy) or progressed to combination therapy (if started on monotherapy) for “period two.” The primary outcome, time to failure, was measured if a patient’s A1c increased above 7% during period one; otherwise, they stayed on period one treatment. The secondary outcome, time to second failure, was measured if a patient’s A1c remained greater than 7% for at least six months during period two. The patient then advanced to insulin treatment during “period three.” Time to first failure determined if there was benefit to combination therapy at the beginning of pharmacological treatment, while time to second failure determined if there was more benefit from initial combined therapy versus sequential additive therapy. Failure is confirmed or denied by a practitioner at a visit every three months, and rate of failure is calculated in each strategy group as a proportion of those failing out of all present in each treatment strategy group.
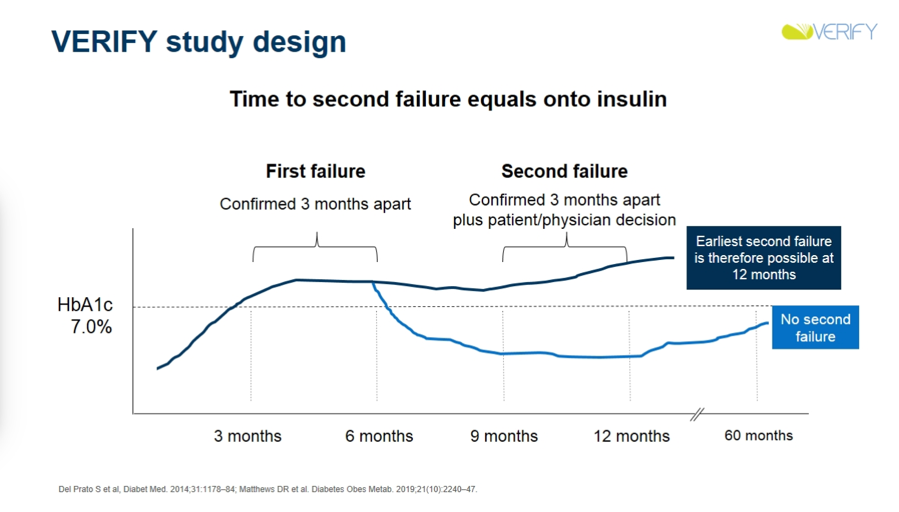
-
Results: Early combination therapy delayed both time to initial treatment failure (median difference in time-to-failure vs. placebo: +25.8 months; HR: 0.51; 95% CI: 0.45-0.58; p<0.0001) and time to second failure (HR: 0.74; 95% CI: 0.63-0.86; p<0.0001). Rate of loss of glycemic control was also lower in the early combination therapy group, and in future studies, will be analyzed to determine beta cell function and insulin resistance. Both treatment options showcased similar success rates, with early combination therapy showing slightly higher success rates that became more pronounced when target A1c amount was lowered. Dr. David Matthews emphasized that this trend means that early combination therapy is more beneficial if A1c goals are lower. Very notably, there did appear to be fewer CV events in the early combination group than in the initial monotherapy group. Of course, VERIFY was not a CVOT, and was not powered for such analyses, making these results exploratory at best. Nonetheless, they are very intriguing, and prompted much discussion during commentary (see below).
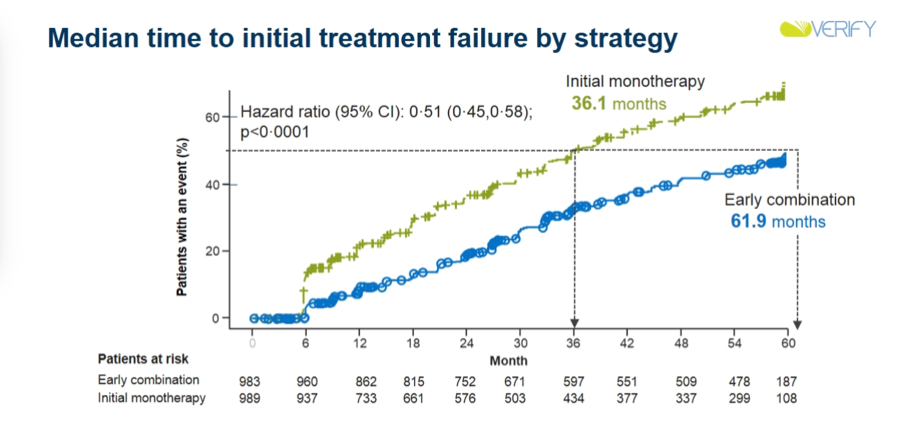
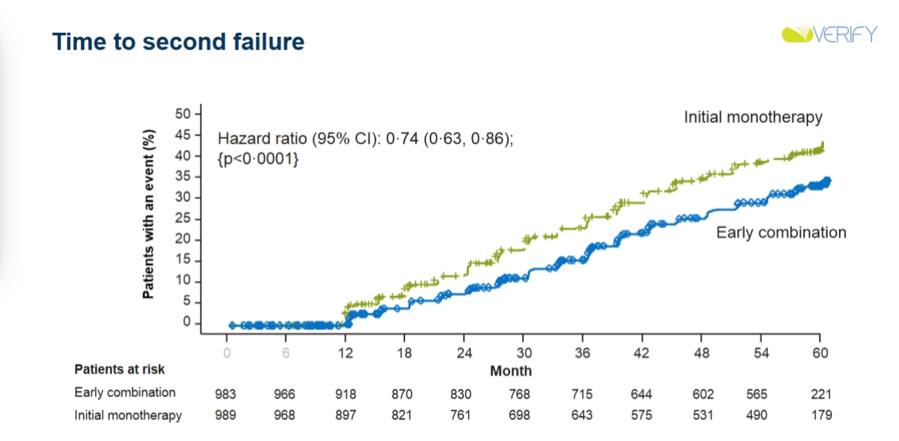
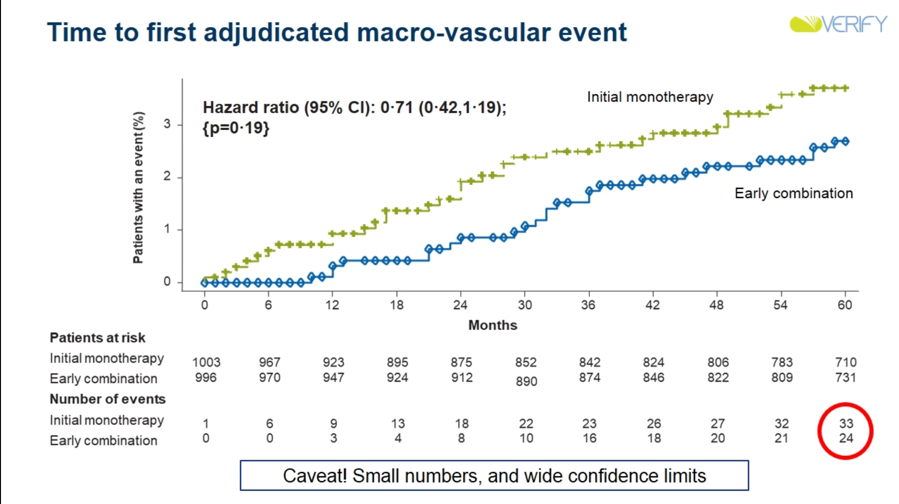
-
Discussing clinical implications of VERIFY, Prof. Stefano del Prato noted that these results could help minimize therapeutic inertia and enhance use of earlier combo therapy. Prof. del Prato also appeared quite bullish regarding potential CV benefits of such a strategy. He emphasized that the finding of fewer CV events in the early combo group is hypothesis-generating only, and certainly not a proof that early combo therapy could decrease CV risk. However, the opening of the two curves (especially at such early time points, as seen in the curves above) surely represent ground for future investigation in his view – we would surely love to see such CVOTs of combo therapies done, especially with combinations of therapies that have proven CV benefits on their own (i.e. SGLT-2s and GLP-1s). In his independent commentary, Dr. Cliff Bailey also honed in on this exploratory outcome of CV events. Dr. Bailey was especially intrigued by the early separation of curves and wonders whether we could see effects in primary prevention, given the relatively healthy patient population enrolled in the trial.
2. CONCLUDE Trial of Tresiba vs. Toujeo in Type 2 Finds No Significant Difference on Primary Endpoint of Overall Symptomatic Hypoglycemia; Exploratory Secondary Endpoints of Nocturnal Hypoglycemia and Severe Hypoglycemia Benefit Trend Toward Tresiba
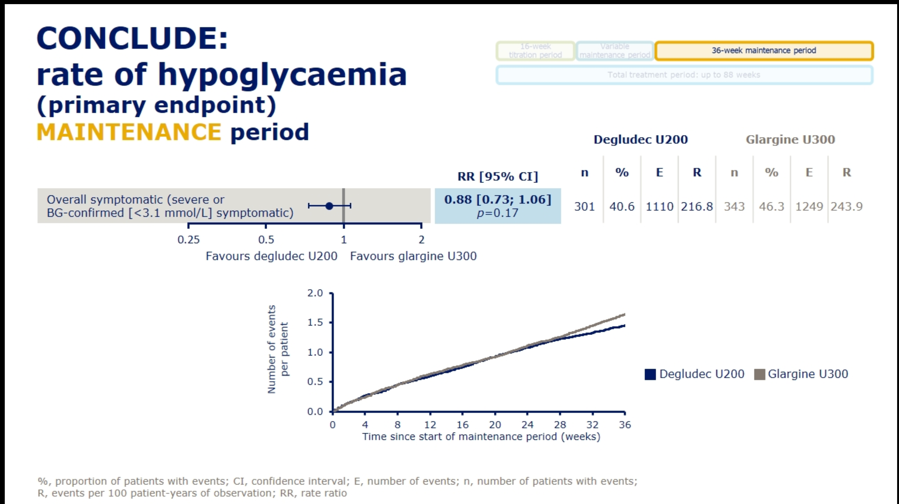
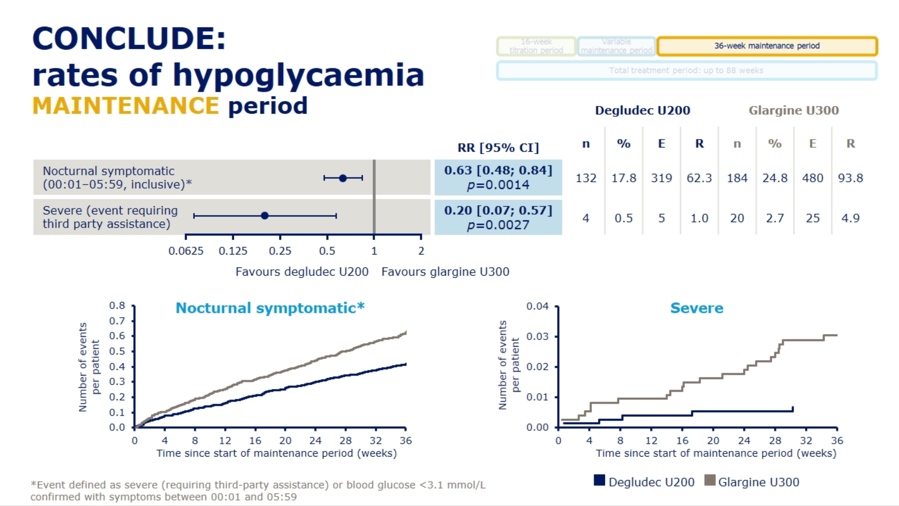
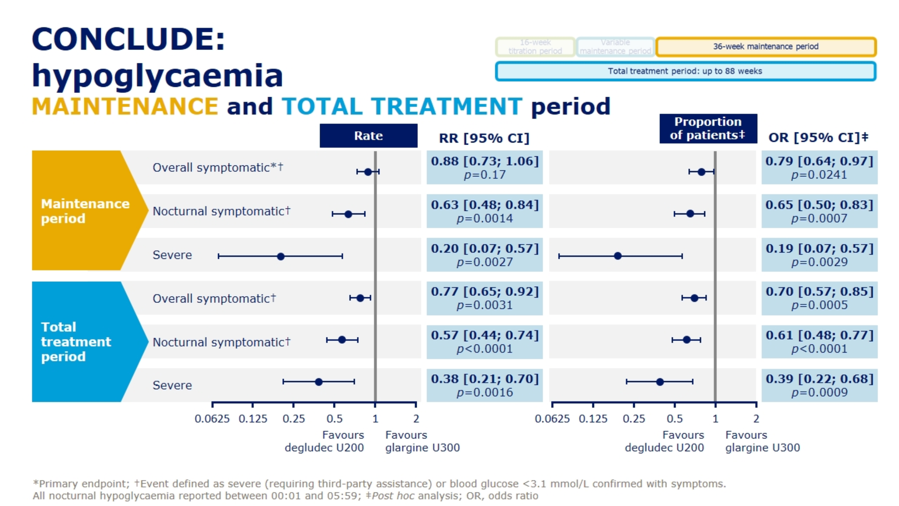
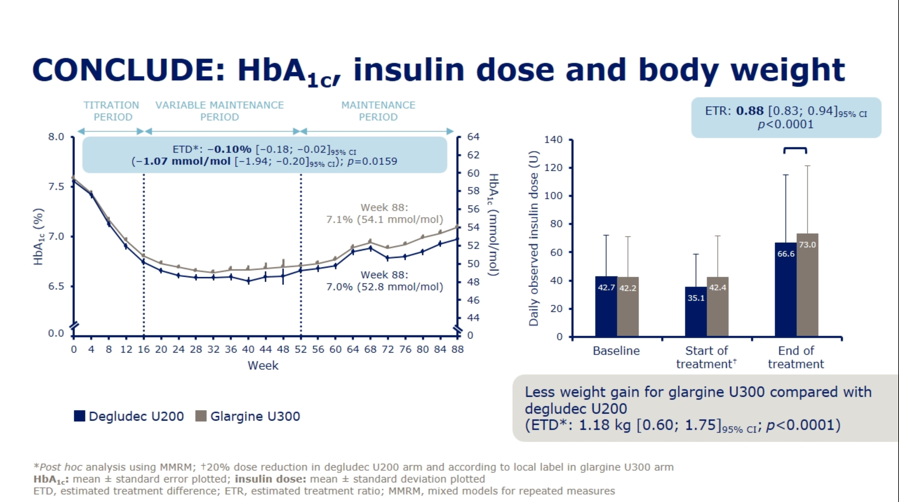
Dr. Athena Philis-Tsimikas presented results from the Novo Nordisk-sponsored CONCLUDE trial (n=1,609) comparing next-gen basal insulins Tresiba (insulin degludec U200) and Toujeo (insulin glargine U300) in type 2 patients, demonstrating no significant differences on the primary endpoint of overall symptomatic hypoglycemia. See slides below for a full rundown of the primary and exploratory secondary endpoints from the trial. For the primary endpoint, rates of overall symptomatic hypoglycemia trended toward lower rates with Tresiba but did not reach significance (RR=0.88; 95% CI: 0.73-1.06; p=0.17). Failure to meet significance on this primary endpoint rendered the following secondary outcome results as purely exploratory and hypothesis-generating: On both nocturnal symptomatic hypoglycemia (RR=0.63; 95% CI: 0.48-0.84; p=0.0014) and severe hypoglycemia (RR=0.20; 95% CI: 0.07-0.57; p=0.0027), Tresiba was associated with nominally significant reductions. Despite their exploratory nature, Dr. Philis-Tsimikas noted that these endpoints might still provide some value in interpretation, explaining that “interpretation of a trial should encompass totality of evidence not just one endpoint.” A more detailed presentation of CONCLUDE is set for EASD Day #4 on Thursday, and we’re looking forward to more commentary on this study.
-
We sensed some pushback during Q&A regarding the interpretation of secondary endpoints in CONCLUDE. One audience member commented: “When interpreting secondary outcomes, we have to interpret them through the primary outcome. If the primary outcome is neutral, all of the alpha has been used up on that analysis. All of the additional outcomes that have been presented are exploratory. I would counter against the conclusions presented here, because the five percent critical alpha has been used up on the primary analysis. This is particularly depressing when we need these comparative clinical trials and we need to have a clear understanding of differences between therapies.” In fact, Dr. Philis-Tsimikas’ presentation of CONCLUDE stressed that interpretation of secondary endpoints must be done with caution and viewed as exploratory and hypothesis generating.
-
For context, Sanofi’s BRIGHT trial of Toujeo vs. Tresiba found overall similar A1c reductions with the two agents, but a predefined subgroup analysis (first presented at ADA 2019 and also at a poster here at EASD 2019) found significantly different mean A1c changes favoring Toujeo at lower baseline eGFR (p-value for interaction=0.02). On hypoglycemia, BRIGHT found a statistically significant decrease in incidence of hypoglycemia <70 mg/dl (OR=0.74, 95% CI: 0.57-0.97, p=0.03) and hypoglycemia <54 mg/dl (OR=0.63, 95% CI: 0.40-0.99, p=0.044) with Toujeo vs. Tresiba during the first 12 weeks of the study – the “titration period.” While there was no significant difference in the latter 12 weeks of the study – the “maintenance period,” from a patient perspective, going an extra three months with more hypoglcyemia if given a choice wouldn’t be the preference. Ultimately, the implication from these data was that Toujeo conferred a 26% risk reduction for hypoglycemia when compared to Tresiba; the p-values of 0.03 and 0.044 were certainly well below 0.05 and the fact that this benefit lasted for 12 weeks was notable. That said, the hypoglycemia benefit disappeared during BRIGHT’s maintenance phase – this deserves further study. While some seemed to think this study was about whether Toujeo is truly superior to Tresiba on hypoglycemia, it was actually far more relevant to use of next-gen insulins in renally-impaired patients – we’d like to study these further. .
-
Overall, we would like to see more evidence pointing to far more people using next-gen basal insulins, full stop – and then, within these populations, examine where notable differences might lie, such as in the case of renally impaired patients. Ultimately, as a community, the focus should be on bringing them to more patients. As Dr. Alice Cheng said at EASD 2018, “I don’t think the big takeaway from BRIGHT is that it demonstrated non-inferiority in terms of A1c reduction between the two next-gen insulins. The big takeaway is that both insulins were able to lower A1c from an average above 8.5% to 7.0% in just 24 weeks.” Notably, it also seems that the conclusion about which insulin is better depends significantly on sub-group analysis: While DELIVER D+ and BRIGHT found no significant difference between the two in hypoglycemia past the first 12 weeks in overall populations, sub-group analysis saw that renally compromised patients may see more benefit related to Toujeo. While the CONFIRM real-world study (n=4,056) suggested that hypoglycemia and treatment discontinuation were more common with Toujeo than Tresiba, we know that both next-gen insulins are associated with far less hypoglycemia than Levemir and Lantus.
3. Dr. Itamar Raz’s Call to Action: “We Should Be Using SGLT-2s in Every Patient w/ A1c >6.5%” – Conversely, Dr. Melanie Davies Urges More Careful Use in Patients Who Can Benefit Most
Dr. Itamar Raz advocated for broader use of SGLT-2s, proclaiming a “Call for Action” in terms of earlier combination therapy with disease modifying drugs and personalized therapy based off of cardiorenal status. In the wake of the DECLARE CVOT for Farxiga demonstrating benefit in a broader patient population than previous trials have, Dr. Raz asserted that “I think we now have enough evidence about efficacy and SGLTs and the need to put these drugs in the forefront of diabetes therapy. Actually, in my mind, we should be using these in every patient with diabetes with an A1c above 6.5%.” We note that although DECLARE showed Farxiga’s consistent benefit across a wide variety of subgroups – including patients without established CVD in terms of hHF benefit – the large NNT for benefit in these patients with treatment might make its efficient use intractable in clinical practice if the value of the benefits isn’t more clearly laid out. In fact, Prof. Stefano Del Prato brought this point up a few months ago at CODHy 2019. Dr. Melanie Davies also touched on this concern in her independent commentary—see below.
-
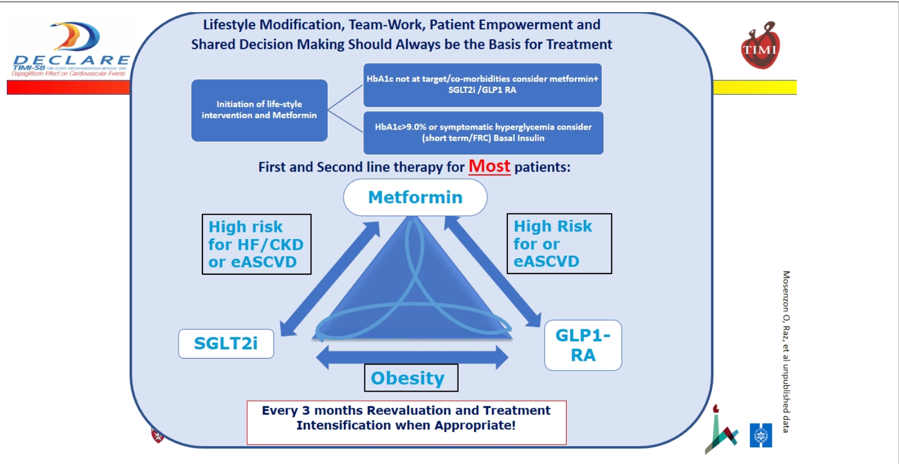
Dr. Raz commented on guidelines for treatment algorithms, with a broad message of simplification so that the average family physician or GP “who has to already follow 50 different guidelines” can easily follow them. On the newly updated ESC/EASD guidelines, Dr. Raz noted that he supports the idea of earlier use of SGLT-2s and GLP-1s, but worries that the guidelines are too “CV and renal” focused – “we also have to focus on the pancreas, and we shouldn’t forget that glucose is also related to macrovascular and microvascular disease.” That was a little surprising to us since earlier use of SGLTs would also help the pancreas even if that is not the primary goal. It’s clear that Dr. Raz thinks that patients early on in the course of type 2 diabetes progression should be prescribed SGLT-2 inhibitors, regardless of the presence of multiple CV or renal risk factors. Presenting his own idea for what the thrust of guidelines should be, Dr. Raz focused on the idea of simplicity with his “SIMPLE” guidelines: Simple, (early) Intervention, Multiple risk reduction, Patient centric/Personalized, Life changing, re-Evaluation. See below for Dr. Raz and Dr. Ofri Mosenzon's proposed guidelines, which he argues are simple, help to avoid therapeutic inertia, and help get patients on SGLT-2s and GLP-1s depending on their personalized cardiorenal status.
-
In an independent commentary, University of Leister’s Dr. Melanie Davies commented on some of Dr. Raz’ points, emphasizing that its “critical that future guidelines target those patients most likely to benefit.” Instead of Dr. Raz’ broad vision of using SGLTs in patient with diabetes with A1cs over 6.5% (we estimate this may be approaching 70% of all patients or higher), Dr. Davies outlined a more measured approach that focused on identifying the patients that may benefit the most from these therapies. We’re hopeful about the potential of new risk stratification tools—such as one presented based off of DECLARE data at ESC 2019—in carrying out this ideal. It would also be terrific to see the expense of therapies compared to the expense of CVD and/or renal disease. Dr. Davies also noted that a new update to ADA/EASD consensus guidelines is in the work to reflect data from DECLARE and CREDENCE, and that the committee is striving to be “evidence based and proactive while also cognizant of the risk/benefit.” We admire Dr. Davies’ commitment to progressive diabetes care and look forward to hearing how the risk/benefit is assessed, particularly long-term benefits.
4. DEFINE-HF: Farxiga Improves KCCQ But Not Primary Endpoint of NTproBNP
In one of the most packed sessions of the day, Dr. Mikhail Kosiborod and Dr. Michael Nassif (both of Saint Luke’s Health System in Kansas City) revealed new results from the DEFINE-HF trial, demonstrating improved Kansas City Cardiomyopathy Questionnaire (KCCQ; symptoms, function, and QOL) at 12 weeks for dapagliflozin vs. placebo in patients with established heart failure with reduced ejection fraction (HFrEF). Crucially, no significant difference in the primary endpoint of N-terminal pro-hormone B-type natriuretic peptide (NTproBNP; a biomarker for HF) was found, so secondary outcome results, including effect on KCCQ, are considered exploratory. These findings further add to the outstanding 26% relative risk reduction (RRR) on CV Death/HHF in HFrEF patients seen in DAPA-HF. Although DEFINE-HF results notably did not generate the “ooh’s and ahh’s” heard at ESC 2019 for DAPA-HF (we speculate that most likely knew the results already, and that was a room filled with cardiologists!), we were excited to see a study specifically aimed at better understanding patient perspectives (KCCQ is a patient-filled questionnaire). Check out the study’s recent publication in Circulation here.
-
Background and rationale. Dr. Kosiborod opened the session with what he described as the “key goals for treating patients with heart failure:” (i) reduce death and hospitalization rate; and (ii) improve health status, including symptom burden, physical limitations, and QOL. Dr. Kosiborod noted that for HFrEF specifically, although there are some treatment options available that reduce death and hospitalization (namely Novartis’ Entresto), ones that reduce symptom burden are essentially nonexistent. As no previous trials have focused on the early effects of SGLT-2 inhibitors on HF-specific health status, DEFINE-HF was designed to fill this need.
-
Inclusion criteria and patient characteristics. Key inclusion criteria included (i) HF for at least 16 weeks (with or without type 2 diabetes); (ii) LVEF ≤40%; (iii) NYHA Class II or Class III symptoms; and (iv) NTproBNP ≥400 pg/mL (or BNP ≥100 pg/mL). Patients were ~60 years of age, obese (BMI ~32), with ~2/3 of patients having Class II HF and 1/3 having Class III HF. Uniquely, nearly 40% of patients were African American, much higher than contemporary HF studies, which we see as a big win for a traditionally underserved population in clinical trials. About 60% of patients had type 2 diabetes (individuals with type 1 diabetes were excluded).
-
Trial design. Following a two-week screening period, participants (n=263) were randomized to either Farxiga/Farxigo (dapagliflozin) or placebo. Participants underwent the intervention for 12 weeks, a relatively short time period.
-
Results. No statistically significant difference was seen in the first primary endpoint of average of 6- and 12-week mean adjusted NTproBNP (HR=0.95; 95% CI: 0.84 to 1.08). On the second primary endpoint of patients that achieve a meaningful improvement in health status (≥5-point increases in average of 6- and 12-week KCCQ overall scores) or NTproBNP (≥20% decrease in average of 6- and 12-week NTproBNP) an HR of 1.8 (95% CI: 1.0-3.1, p=0.0386) favoring dapagliflozin was found. Although Dr. Kosiborod warned of very small sample size during subgroup analysis, of note, there was no difference in the results between (i) those with or without diabetes; or (ii) different types of RAAS treatment (ACE/ARB, ARNI, or neither). In terms of memorable secondary endpoints, there were significant improvements in KCCQ total symptom, physical limitation, and quality of life scores but not social limitation score. NNT for overall KCCQ was just 10. No significant change was seen in 6-minute walk test, A1c, or weight. Safety events were well-balanced in each group.
- During Q&A, Dr. Kosiborod remedied the inconsistencies between KCCQ and 6-minute walk test results by noting that KCCQ is much more specific to HF-related limitations. He commented that the 6-minute walk test can also be affected by study coordinator, as well as non-HF-related limitations such as “a knee hurting, having a cold, or not sleeping well.” In general, he pointed out that strong correlations between KCCQ and 6-minute walk test have not been recorded.
5. SGLTs in Type 1: Are We Being Overcautious + Not Considering Potential CV and Renal Benefits? Impassioned Calls for a Type 1 CVOT Grow!
Dr. Thomas Danne gave a passionate and powerful overview concerning the safe use of SGLTs in type 1 diabetes. SGLTs in type 1 have been discussed at length over the past several years, and we were very interested to see Dr. Danne highlight several new initiatives in his talk since the approval of SGLTs in Europe earlier this year. Notably, these are aimed at improving safe use of these efficacious agents, with many major points dovetailing nicely with his similar recent talk at Keystone 2019.
First, Dr. Danne reviewed several of the published risk mitigation strategies that have emerged recently: these include the STICH Protocol published by Dr. Satish Garg and colleagues, the International Consensus document spearheaded by Dr. Danne and his own group, and the recently published STOP DKA protocol authored by Drs. Irene Hramiak, Bernie Zinman, and others. Excitingly, he also touched on multiple new efforts being undertaken on this front: (i) the EASD e-learning module on adjunct type 1 therapy; (ii) ATTD education portal for industry education on the subject; (iii) the KetoAWARE program for structured DKA education in type 1; (iv) several HCP-focused DKA education programs; and (v) JDRF’s call-in requesting research grant proposals investigating safe use of SGLTs in type 1 diabetes. Broadly speaking, we’re enthusiastic about how this emphasis on DKA prevention is spilling over into broader efforts toward DKA education and risk mitigation, and are hopeful that with this focus and education to both HCPs and patients that SGLTs can truly deliver its potential benefits to a wide range of people with type 1. Of course, so many PCPs do see people with both type 1 and type 2 diabetes and we would also like to make sure that education is a requirement and not optional for both groups.
-
Dr. Danne opened his talk with an impassioned, off-script plea to not be over cautious to the point of withholding important benefits to patients: “I’m a little bit angry. I completely understand with all the data that we have that we need to be cautious. But we also have situation where people are starting to be over cautious. Tell me, if you would have a child with type 1 diabetes – at age 10, and you look through the data of the Swedish Diabetes Registry – you realize that your child will lose 17 years of life (if a girl) or 14 years of life (if a boy), and you know that this loss of life is due to increased CV mortality risk. Yes, there is a risk of DKA, and yes we are doing a bad job so far, and yes I hope we will do a better job – but why are we so cautious that we have not yet started trials in this very vulnerable pediatric population with a drug that has shown positive CV mortality and renal outcomes effects in type 2 diabetes and in those without diabetes? Is it so difficult to believe that they will have similar benefits in type 1 diabetes?”
-
We couldn’t agree more with Dr. Danne’s passionate call to better study potential CV and renal long-term outcomes associated with SGLTs in type 1s, as we believe this to be a major consideration in the risk/benefit profile of this therapy that has not been assessed (adequately or at all). Earlier on in this same session, Dr. Thomas Pieber similarly called for CV and renal outcomes studies for SGLTs in type 1 diabetes: “I think we have to do an outcomes trial, a well-designed trial that looks into the implications of SGLT-2 inhibitors in long term outcomes for CV and renal outcomes in type 1 diabetes.” Of course, Drs. Danne and Pieber are surely not the first thought leader to emphasize the need for a type 1 CVOT. At EASD 2018, we heard Dr. Julio Rosenstock echo a similar sentiment, saying that “if there was ever a need for a pragmatic CVOT, this is it – SGLT inhibitors in people with type 1 diabetes at high risk for CV disease.” Although it would make such a trial more expensive, we believe those at “normal” CV risk should also be studied. At ADA 2018, Dr. David Cherney offered an analogous call for a cardio/renal outcomes trial in type 1 diabetes, and Dr. Anne Peters shared that many of her type 1 patients ask for an off-label SGLT-2 inhibitor primarily for the potential cardioprotective benefits – this is clearly a topic on people’s minds, from patients to providers to KOLs. To be sure, we currently have no hard evidence on organ protection with these agents in type 1, though the mechanisms of SGLT inhibition – which are not thought to be glucose-mediated – should theoretically play out in type 1 diabetes much like they do in type 2 – see Dr. Per-Henrik Groop speak on this this topic, from ATTD 2019 for more.
-
-
Dr. Danne is also hopeful that EMA will eventually ease its current BMI restriction and open up SGLT therapy to most patients with type 1. Currently, both dapagliflozin and sotagliflozin have been approved by EMA, but with the stipulation that it can only be used in people with a BMI ≥ 27 – we also learned at EASD that there is a requirement (minimum dosage) of daily insulin intake. Dr. Danne noted that he “likes the idea of caution, but we now need to see if our risk mitigation strategy works in the real world. Then, I think EMA will reconsider their original guidelines and approve this drug for those with normal body weight as well.”
-
Dr. Danne firmly believes that DKA risk can be mitigated to the point of achieving lower rates of DKA than seen in the many clinical trial programs of SGLTs in type 1. We agree here, and are excited to see real-world evidence collected with Farxiga/Forxiga in type 1 patients in Europe once it is fully rolled out.
6. Additional Commentary on Positive Renal Data from DECLARE: Consistent Effects in Patients with Relatively Normal Kidney Function Emphasized
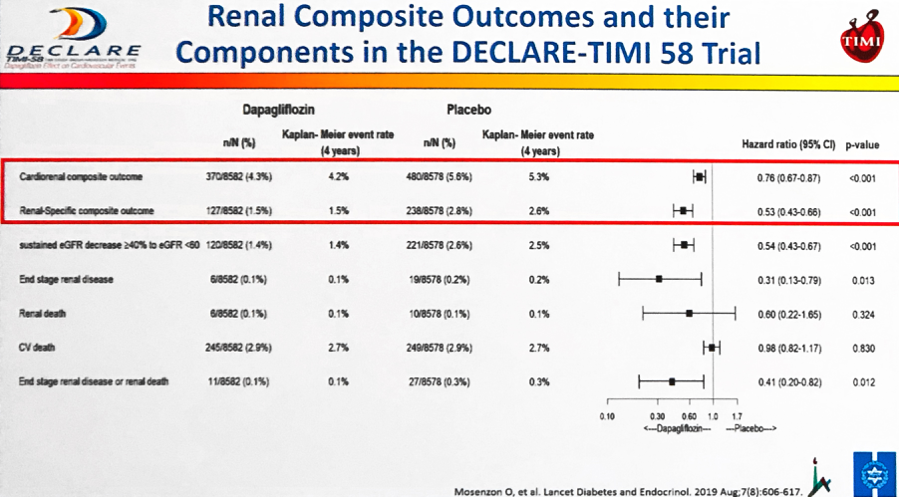
Ofri Mosenzon (Hadassah Medical Center) gave the positive renal outcomes from DECLARE, specifically focusing today’s talk on the drug’s renal benefit even in participants with relatively normal kidney function. As a reminder, dapagliflozin conferred both (i) greater improvements in UACR and (ii) lower rates of UACR deterioration vs. placebo – demonstrating the drug’s potential in both CKD prevention and treatment for patients with type 2 diabetes. In today’s talk, Dr. Mosenzon specifically drew attention to dapagliflozin’s positive renal effects in the DECLARE cohort with relatively robust kidney function in terms of UACR (<30 mg/g) and eGFR (>60 mL/min/1.73 m2). She noted that even with more than 50% of participants having not just normal albuminuria, but either undetectable or albuminuria <15 mg/g at baseline, there were significant improvements in (i) renal-specific composite outcomes; (ii) sustained eGFR decrease ≥40% to eGFR <60; (iii) ESRD; and (iv) renal death (slide below). Dr. Mosenzon emphatically pointed out that for the renal-specific composite endpoint, there was “absolutely no relation to” baseline UACR and stated that dapagliflozin is effective on renal specific outcomes at all baselines UACR.
-
Dr. Melanie Davies agreed during independent commentary that there is no relation between baseline UACR and the renal-specific composite endpoint and urged the crowd to also consider the high NNT values in the low eGFR and high UACR individuals (NNT reaching up to 1250 in participants with the highest renal function). All in all, Dr. Davies emphasized making intentional choices about which populations will most benefit from treatment – we thought this was a very thoughtful qualification to make amongst the swath of positive renal DECLARE data, and needless to say, we also hope that this is happening continuously in medical care (not just related to SGLTs)!
7. Ellipse Trial Shows Encouraging Results for Liraglutide in Adolescents with Type 2 Diabetes
University of Birmingham’s Dr. Timothy Barrett presented results from the Ellipse trial, demonstrating the efficacy of GLP-1 agonist liraglutide as an add-on to metformin in adolescents with type 2 diabetes (n=134). After 26 weeks, from a baseline A1c of 7.5%, average A1c fell 0.64% with liraglutide vs. a 0.42% rise in A1c with placebo (p<0.001). This difference persisted at the 52-week mark: A1c reductions with liraglutide stood at -0.50%, vs. +0.80% with placebo (p<0.001). Liraglutide also produced improvements in other key secondary outcomes, including FPG (-19 mg/dl vs. +14 mg/dl with placebo at 52 weeks) and proportion of patients who attained an A1c <7% (64% vs. 37% with placebo at 26 weeks – this is quite a striking difference and we wish we could see full Time in Range data for at least part of the study). Over the full study period, the most common adverse events (found in 21-29% of liraglutide-treated patients) were nausea, vomiting, and diarrhea, as is seen in patients taking GLP-1 agonists, particularly when not titrated optimally. As for hypoglycemia, there were more symptomatic (19 vs. 6) and asymptomatic (21 vs. 12) events in the liraglutide-treated group vs. the placebo-treated group (significance could not be tested due to the small number of events). This was surprising to us, but during Q&A Dr. Barrett elaborated that the majority of these hypoglycemia events occurred in patients who were additionally taking insulin (one of the only medications approved for adolescents with type 2 diabetes). Overall, the Ellipse results are encouraging for the use of liraglutide in this under-served patient population. In the ensuing Q&A, Dr. Barrett affirmed that the results of this trial will change his own clinical practice. In his words, “I’d much rather use liraglutide than insulin.” As a reminder, FDA approved Victoza in this patient population in June 2019.
-
At ADA 2016, we were warned that the incidence of early onset type 2 will increase in the coming years, especially in underserved minority groups. This adds additional urgency to the need for therapies beyond metformin and insulin in this patient population, especially given the increased risk of especially aggressive diabetes complications with such an early onset of disease. We would imagine that liraglutide’s CV benefits could be especially meaningful for this population as well though teens were not studied in the CVOTs as far as we know.
8. A Role for GLP-1 Agonists and GLP-1/GIP Dual Agonists in Treating Alzheimer’s and Parkinson’s?
Henan University’s Dr. Christian Hölscher discussed early evidence that incretin analogues such as GLP-1 agonists and GLP-1/GIP dual agonists could be neuroprotective in Alzheimer’s and Parkinson’s diseases. In rodent models of Alzheimer’s disease, both Sanofi’s Lyxumia (lixisenatide) and Novo Nordisk’s Victoza (liraglutide) improved memory, boosted synaptic plasticity, decreased the load of beta-amyloid plaques in the brain, and reduced the number of activated microglia (suggesting an anti-inflammatory mechanism). Similarly, in rodent models of Parkinson’s disease lixisenatide and liraglutide improved motor activity, preserved neurons in the substantia nigra (the main area of damage in Parkinson’s disease), and reduced activated microglia (again, pointing to anti-inflammation). On the heels of these results, in-human trials of GLP-1 agonists in Parkinson’s disease are underway. A 2013 trial showed improvements in motor skills and cognitive performance with AZ’s Bydurdeon (exenatide) in people with Parkinson’s disease, and these lasted three months after the drug treatment was stopped. Three additional phase 2 trials are currently underway examining liraglutide (completion expected in 2019), lixisenatide, and semaglutide. Dr. Hölscher showed additional data demonstrating that GLP-1/GIP dual agonists have an even more powerful effect on rodent models of Alzheimer’s and Parkinson’s diseases. He has developed two novel GLP-1/GIP dual agonists that are able to cross the blood-brain-barrier (not true for all diabetes drugs, particularly peptides) and founded a new company, Kariya Pharmaceuticals, to advance these candidates through toxicology and dose-response studies in humans.
-
Why the beneficial effect for incretins? Dr. Hölscher noted that incretins normalize energy utilization, reduce inflammation, and enhance cell repair. In the brain, this could translate to protection against neurodegenerative disease.
-
Although these results are incredibly early-stage, needless to say we are extremely intrigued and eagerly look forward to watching the first in-human trials. Dr. Hölscher noted that type 2 diabetes is a hypothesized risk factor for both Parkinson’s and Alzheimer’s, so these findings could add one more item to the (long) list of diabetes complications that are lessened with GLP-1 agonists.
Diabetes Technology Highlights
1. DCCT/EDIC Cohort Wearing FreeStyle Libre Pro: Only 9% Achieved >70% TIR Target, Up to 19% in Those With Automatic Glucose Suspend
The afternoon session, “Benefits of Technologies in Type 1 Diabetes,” was full of learning, including a fascinating study of DCCT/EDIC participants wearing FreeStyle Libre Pro for (n=765). Dr. Rose Gubitosi-Klug (Case Western Reserve) shared an analysis of CGM profiles from the DCCT/EDIC cohort and compared them to the new time-in-range guidelines. The study enrolled 765 participants (!) who wore the blinded FreeStyle Libre Pro CGM for a mean of ~12 days. Of the group, 58% used insulin pumps, 27% routinely wore real-time CGM, 21% routinely were on CGM and pump, and 11% had CGM + pump with automatic insulin suspension turned on. The group had a mean age of 59 years and diabetes duration of 37 years. Aggregate time-in-range for these participants was ~52% with a mean glucose of 170 mg/dl. Only 9% of participants hit the >70% TIR goal, only 30% achieved the <4% time <70 mg/dl target, and only 23% hit the <25% time >180 mg/dl. Outcomes for the sub-group on insulin suspend (presumably Tandem’s Basal-IQ or Medtronic’s 670G) were slightly better, with 19% achieving >70% TIR, 40% spending less than 4% of time <70 mg/dl and 35% spending <25% of time >180 mg/dl. Even still, this reinforced the takeaway we had earlier this year when Abbott shared global data on FreeStyle Libre users – most are not close to hitting the >70% time-in-range benchmark and there are undoubtedly many interventions that could be helpful – therapeutic and otherwise – and there may well be “inertia” in those that are advising these patients. While some would point out that CGM is a tool, not a therapy – we also would certainly believe that there is far more potential than is being harnessed in this group to use this data to drive therapeutic change. Indeed, doctors and others (including patients) could use the data more optimally on the intervention front. Using the data to drive changes in medication, food, and exercise is the key, and is an enormous area of opportunity, particularly as the field drives toward automating insulin delivery.
|
Target |
Overall % achieving target (n=765) |
Insulin suspend % achieving target (n=85) |
|
>70% time-in-range (70-180 mg/dl) |
9% |
19% |
|
<4% time spent <70 mg/dl |
30% |
40% |
|
<1% time spent <54 mg/dl |
28% |
47% |
|
<25% time spent >180 mg/dl |
23% |
35% |
|
<5% time spent >250 mg/dl |
17% |
32% |
-
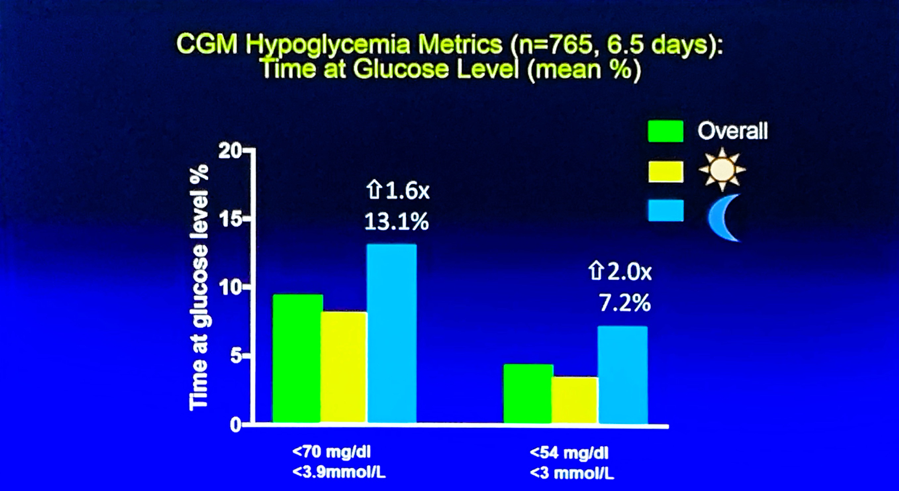
Hypoglycemia continued to be an issue in this large study of longstanding type 1 diabetes, particularly at night. Percentage of time spent <70 mg/dl at night was 13%, compared to ~8% during the day, while percentage of time spend <54 mg/dl at night was 7%, compared to ~3% during the day. A caveat is in order here, of course: FreeStyle Libre Pro does tend to overread hypoglycemia (per the label), so the true outcomes may be slightly better. As expected, time-in-range during daytime (6 AM – midnight) and nighttime (midnight – 6 AM) were nearly identical (51% during the day vs. 52% at night), but mean glucose was significantly lower at night (173 mg/dl during the day vs. 159 mg/dl at night), obviously due to insulin “matching” carbs being closer at night. We commend the researchers for taking on this work.
2. COMISAIR Full Three-Year Data (n=94): CGM Drives Improved Glycemic Outcomes Regardless of Insulin Delivery Method in Type 1s; Fewer Hypoglycemia Episodes and Five Increased Time-in-Range
Charles University’s Dr. Jan Šoupal presented full three-year results from the COMISAIR study (n=94 type 1 adults), demonstrating the huge benefits provided by CGM, including five hours increased time-in-range, >1% A1c reduction, and fewer episodes of hypoglycemia, regardless of insulin delivery method. The prospective, real-world, investigator-initiated study is the longest CGM trial ever conducted, evaluating: (i) SMBG + MDI (n=21); (ii) SMBG + pump (n=25); (iii) Dexcom CGM + MDI (n=22); and (iv) Dexcom CGM + pump (n=26). At ADA 2019, Dr. Šoupal shared the topline data: time-in-range increased from 49% to 69% in the CGM + MDI group and increased from 51% to 72% in the CGM + pump group (improvements of ~5 hours/day); time <70 mg/dl decreased from 9.4% to 5.5% in the CGM + MDI group and 9.0% to 5.3% in the CGM + pump group (declines of nearly one hour); and A1c decreased by 1.3% and 1.2% for the CGM + pump and CGM + MDI groups, respectively (baseline A1c: 8.2%). For the first time, we Dr. Šoupal also shared data on hypoglycemic and ketoacidosis episodes. The study recorded seven total severe hypoglycemia episodes, with five occurring in the SMBG groups, compared to just two in the CGM groups. Notably, one of the episodes in the CGM groups actually occurred when the participant was not wearing the sensor (meaning just one episode in the CGM group vs. six without CGM on). The study only recorded three ketoacidosis episodes. According to Dr. Šoupal, the results will be published in Diabetes Care in November.
- The main takeaway from the three-year data supported one-year results (Šoupal et al., 2016) presented at EASD 2016: adding CGM to MDI drove similar glycemic outcomes as adding CGM to a pump. Notably, these improvements appear quickly and have been sustained over three years. See our report from ADA 2019 for a full rundown of the data.
3. GOLD Post-Hoc Analysis: CGM Delivers Benefits Regardless of SMBG Frequency; Jaeb’s Kellee Miller Recaps WISDM Trial
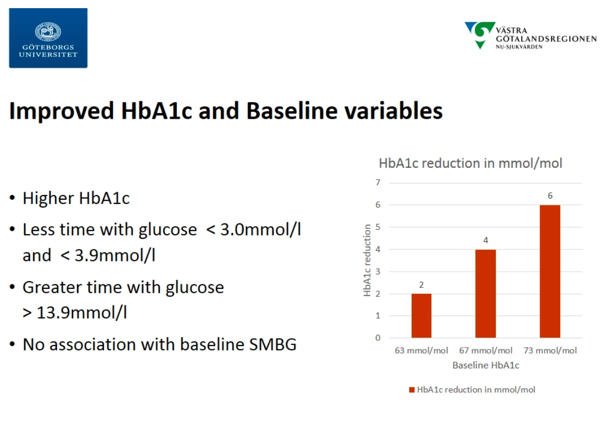
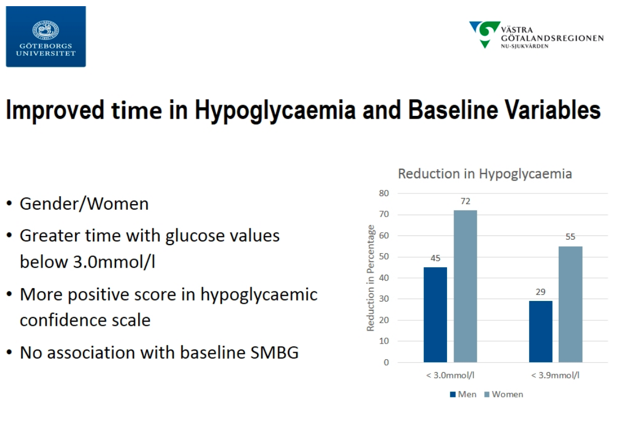
Continuing the CGM-focus during the “Benefits of Technologies in Type 1 Diabetes” sessions, Dr. Arndís Ólafsdóttir (University of Gothenburg) presented a post-hoc analysis of data from the GOLD study, finding that the benefits delivered by CGM were independent of SMBG frequency. The Swedish GOLD study (n=161) was published in JAMA in 2017, one of the early studies to build the evidence-base for CGM in MDI. The study showed an A1c reduction of 0.4%, a time <70 mg/dl reduction of 29 minutes/day, and satisfaction and well-being improvements. In the post-hoc analysis, the group attempted to categorize variables that influenced CGM effectiveness. CGMs were found to be effective across all baseline A1c levels. Notably, the analysis found that SMBG frequency had no influence on CGM effectiveness. This could be particularly important for payers, such as Medicare, which currently requires users to take four fingersticks/day for 90 days for CGMs to be reimbursed and is perceived as a giant hassle for many in getting CGM in the first place. Lastly, the analysis found significant influence of gender on CGM effectiveness, with women seeing greater improvements than men. We have not heard that before and wonder why that might be … we are curious if they are wearing it for a higher percent of time.
-
Jaeb Center’s Dr. Kellee Miller gave a quick 15-minute recap of the WISDM trial (first read out at ADA 2019), which showed significant benefits delivered by Dexcom G5 CGM in 203 older adults (≥60 years) with type 1 diabetes in every outcome measured. For the primary outcome, time <70 mg/dl, the adjusted treatment group difference for the CGM group was -1.9% (p<0.001), amounting to a difference of 27 minutes/day. There was also a significant between-group difference in time <54 mg/dl, with the CGM group showing an adjusted difference of -1.0% (-14 minutes/day) (p<0.001). Time-in-range (70-180 mg/dl) improved significantly for the CGM group, with an adjusted difference of +9% (+2.1 hours/day) (p<0.001). Time >180 mg/dl and >250 mg/dl also decreased for the CGM group, with adjusted differences of -6% (-1.4 hours/day; p<0.001) and -4% (-58 minutes/day; p<0.001), respectively. Accordingly, A1c significantly improved in the CGM group, dropping by 0.4 percentage points (baseline A1c: 7.6%) and amounting to an adjusted difference of -0.3% (p<0.001). See our full analysis of the results from our ADA report. We hope that this data helps further support the outstanding benefits of CGM in the Medicare population, including a need for fewer restrictions around fingerstick documentation.
4. NHS Data shows A1c Reduction of 0.6% on Pump Initiation (n=505 Type 1s) After Five Years, ~2% Reduction for Baseline A1c ≥10%
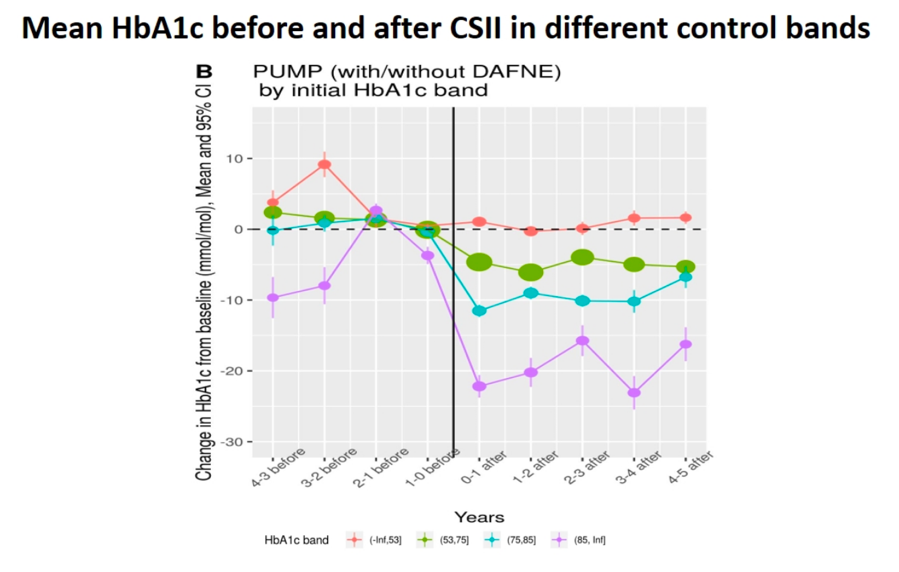
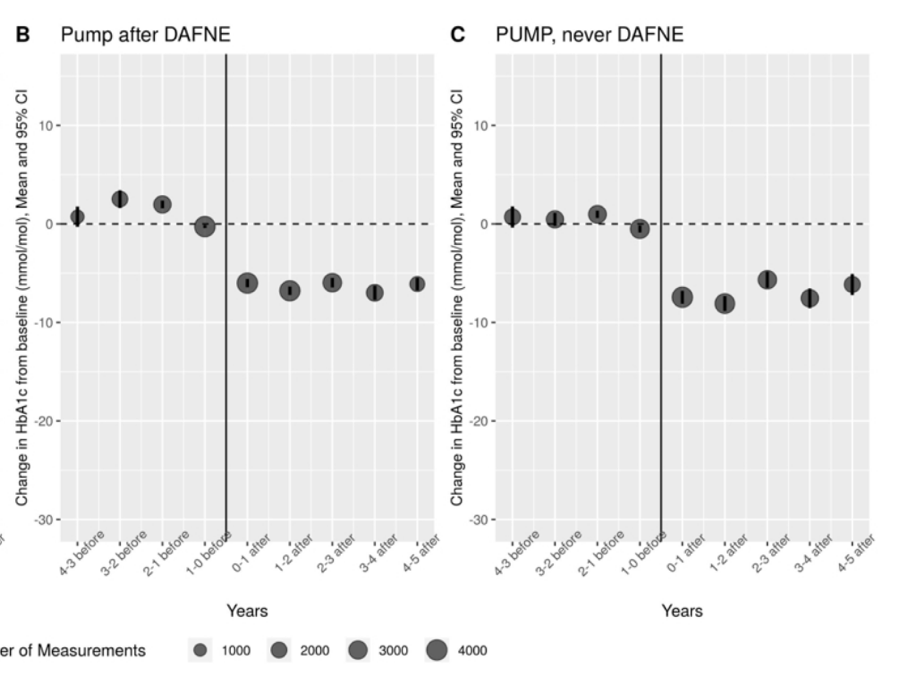
Dr. John McKnight (Edinburgh Centre for Endocrinology and Diabetes) shared results from a retrospective analysis of patients initiating pump therapy (n=505), finding an immediate and sustained 0.6% A1c reduction after five years. All data was collected from patients with type 1 diabetes who were seen in NHS’s Lothian region. As expected, improvements in A1c were greatest for patients with highest baseline A1c, with patients ≥10% A1c at baseline seeing a ~2% A1c reduction after one year. Conversely, patients who began pump therapy with A1c ≤7% did not see any significant changes in A1c over time. Notably, the study found little effect of age, gender, or diabetes duration on the effectiveness of pumps. Additionally, researchers looked at whether the standardized, five-day diabetes nutrition course “Dose Adjustment for Normal Eating” (DAFNE) would change the effectiveness of pumps. Comparing patients who went through DAFNE before initiating a pump (n=208) with those on pumps and no DAFNE training (n=297) showed no significant effects of the course on pump effectiveness. We would note however, that during Q&A, Dr. McKnight guessed that non-DAFNE pump users still received “10-15 hours” (!) of carb counting training that was well-standardized throughout Lothian. Note in the chart below, A1c is reported in mmol/l.
-
The REPOSE trial (Diabetes UK 2016) found an identical 0.6% A1c reduction in pump users (n=132) who went through the DAFNE course after two years. Additionally, REPOSE found that DAFNE-trained MDI users (n=132) also saw a 0.6% A1c reduction after two years. That trial seemed to suggest CSII may not have significant advantages over MDI with proper training, though we would note that pump users did report higher treatment satisfaction and lower “daily hassle” than the MDI group. Dr. McKnight did note that CGM began to enter NHS Lothian ~18 months ago, and may be more effective than pumps at improving glycemia, which they are “just now looking into” – we would hope that CGM would help drive, at minimum, effective therapeutic and nutritional interventions.
5. Severe Hypo Elevates Risk of Future Mortality in Type 1 Welsh Cohort from 2000-2018
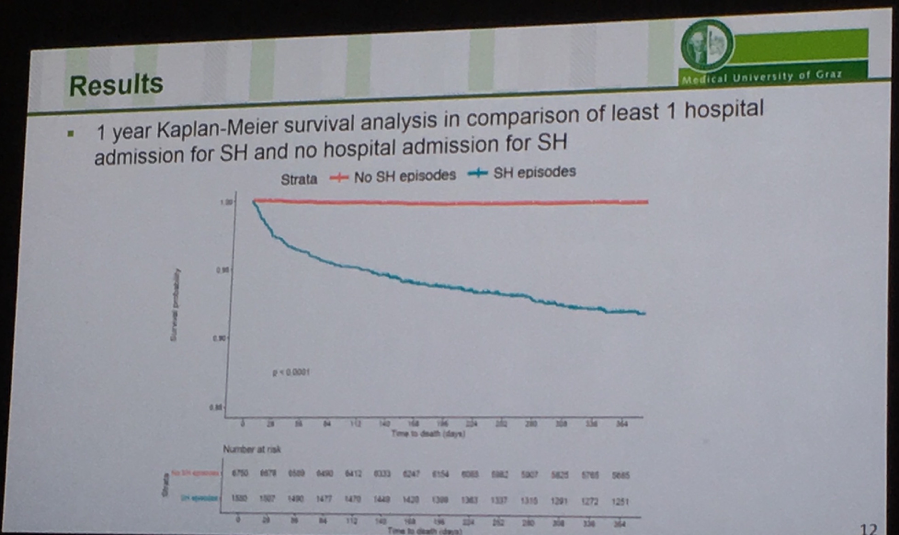
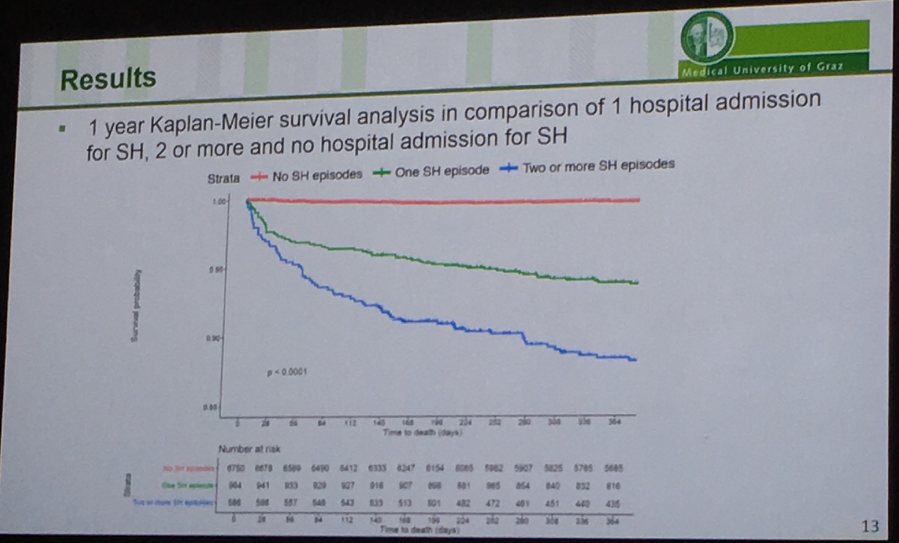
Medical University of Graz’s Dr. Othmar Moser presented a retrospective cohort analysis of type 1s in Wales between 2000-2018 confirming that severe hypoglycemia is an important marker for mortality risk. The Kaplan-Meier curves below show survival out to one year following a hospital admission for severe hypoglycemia in the group of 8,626 people with type 1 diabetes (mean age 32 years; mean A1c ~8.9%). 18% of the cohort had at least one severe hypoglycemia during the 18-year-period; 9% of the group passed away, and 27% of those who died had a preceding hospital admission for severe hypoglycemia. Note that in the K-M curves the y-axis extends down to 0.85, and the data set does not capture severe hypos that were treated at home. The curves clearly depict that any severe hypoglycemia resulting in a hospital admission is worse than no severe hypoglycemia in terms of mortality (obvious), and 2+ severe hypoglycemia episodes is worse than just one (also obvious). Digging deeper, those who died in <10 days following a severe hypo experienced on average more severe hypos during the study period (mean 2.3 episodes) than those who died >10 days after a severe hypoglycemia (mean 2.1) and those who were alive after their severe hypo (mean 1.8) (p=0.005). Further, those who survived had slightly higher A1cs (mean 9.2%) vs. those who died >10 days (mean 8.9%) and <10 days (8.1%) following a severe hypo. There’s clearly room to more aggressively lower mean glucose in all these populations, provided there is use of CGM to avoid severe hypoglycemia and to direct better therapeutic and other interventions.
-
Based on Dr. Roy Beck et al.’s retrospective analysis of DCCT fingerstick data, frequency of biochemical hypoglycemia predicts future severe hypoglycemia in type 1s. Taken together, these studies again affirm that minimizing biochemical hypoglycemia should be a primary goal to reduce the risk of severe hypoglycemia (and the latter’s link to mortality).
Selected Questions and Answers
Prof. Simon Heller (Sheffield): As you know, the whole field agrees there’s an association between severe hypoglycemia and mortality, and of course we should reduce severe hypoglycemia. The controversy is whether it’s causal or not. In a type 1 population who are arguably less frail, it may be that there are less confounding comorbidities. Can your data shed any light on this discussion?
A: That’s absolutely right, the discussion is ongoing. I think it’s quite interesting that mean age was 32 years, this might be further info that it might be a causal relationship because we don’t expect such massive comorbidities in this young group. To draw a final conclusion though, it might be difficult to say there is a causal relationship. In my opinion, though, I think we’re getting closer to determining that it is a causal relationship.
Q: I wonder whether you have info on which patients used CGM?
A: We do have this info, but it’s quite difficult to quantify. The study was from 2000-2018, and in early 2000, not everyone was using CGM, so we can’t forget these results might be performed without massive influence of CGM. But we’re working to get that info.
Exhibit Hall
Diabetes Technology
Abbott
Abbott’s trademark bright yellow was visible throughout the Fira Barcelona Gran Via conference center as reps handed out yellow tote bags featuring a big “In the Zone” graphic on one side and the FreeStyle Libre butterfly on the other. Two sides of Abbott’s sizeable booth were dedicated to its data-integration collaboration with Novo Nordisk’s connected pens, NovoPen 6 and Echo Plus (announced in February) – the first we can ever recall seeing smart pens in Abbott’s booth! Abbott also announced a collaboration with Sanofi earlier this week, though there was no mention of it that we could find in the booth. (Novo Nordisk separately announced a smart pen collaboration with Medtronic.) Abbott representatives were also on hand to demonstrate the new FreeStyle Libre 2 system (Bluetooth, optional high and low alarms, same price and form factor), which has officially launched in Germany and Norway to lots of enthusiasm. As a reminder, FreeStyle Libre 2 is still under review in the US. We loved the “Free to Dream” marketing graphic that highlighted the optional alarms enabling patients to sleep in peace.



A. Menarini Diagnostics
A. Menarini’s booth showed off the CE-Marked GlucoMen Day CGM, “powered by Waveform.” This is the WaveForm CGM (previously branded “Cascade 1”) for which A. Menarini is set to perform sales, marketing, training, and customer support for WaveForm’s CGM in territories throughout Europe, the Middle East, Africa, and Latin America. The system is now CE-Marked – in line with expectations for 2019 – and is expected to roll out “all over the EU community” starting in November. In its current state, the system has 14-day-wear, “MARD of 11% or less” per a rep (though a DTM presentation put MARD closer to 13%), and requires one fingerstick calibration per day. Reps were excited about the reusable one-button-press inserter, which has to be manually loaded but generates less waste than Dexcom’s G6 and Abbott’s FreeStyle Libre. The system doesn’t have a dedicated receiver (i.e., data goes straight to an app that wasn’t on display) and it will come with a Bluetooth meter for calibrations. A rep told us he expects the CGM will be available in the US in 2020, which could align with previous expectations of a December 2019 FDA filing. See our DTM coverage for a recent rundown of WaveForm’s study progress, accuracy, and product features, and pictures. It’s still unclear whether this CGM will be competitive in Europe or the US – will it be able to compete with Dexcom and Abbott on price and feature set?

Dexcom
Dexcom’s booth advertised the G6 CGM and Clarity data management applications. On the international side, the most recent news was G6 becoming available for order in Canada earlier this month. Notably, to meet global demand, Dexcom is doubling G6 manufacturing by the end of the year, and its 2Q19 results certainly suggested robust growth across the world. When asked about possible new G6 features and anticipated launches of the G6 Pro and G7, no further information could be provided at this time – G7 is certainly very highly anticipated. Information on rollout of the company’s low-cost G6 transmitter that is needed for its direct-to-Apple Watch data transmission was not given. The G6 will be launched into Medicare in 4Q19.
EyeNuk
EyeNuk demoed its EyeScreen Human+AI Diagnostic Service that utilizes AI for detection of diabetic retinopathy. The company representative mentioned that in a cohort of multi-ethnic patients living in Los Angeles, it had a ~91% sensitivity and specificity – we haven’t yet seen published data. The device is currently available in Europe and Africa and is also expected to get FDA clearance in the next six weeks. For context, Iowa-based IDx-DR was the first AI-driven diabetic retinopathy detection device cleared in 2018, and there are others in development (e.g., Verily/Nikon, IBM Watson Health). A clinical study (n=900) of Idx-DR showed the device to have 87% sensitivity and 90% specificity. The price for EyeNuk’s technology has not been established, but the representative mentioned that reimbursements in France are ~$60/use. Whether or not patients themselves or physicians will be able to operate it is dependent on legal rules on a country-by-country basis. Notably, it works on both the dilated and undilated eye.
-
On the company’s website, new products in the tech pipeline include an EyeMark device, which can automatically track diabetic retinopathy progression and an EyeApp smartphone application, which allows providers to conduct DR exams from any location.
-
This field has no shortage of technologies and companies, though we have not heard anything on commercial uptake – how many automated systems for eye screening are currently available worldwide? Do healthcare providers like them? Do they save time? How long does it take for them to pay for themselves – e.g., in save time, more efficiency, etc.?
LifeScan
The OneTouch-branded booth advertised the new “Insulin Mentor” feature in the OneTouch Reveal app, a prescription-only bolus calculator with an integrated food library. It appears that anyone can use the app, but they must get a prescription to unlock the bolus calculator. We’d note that LifeScan’s OneTouch Reveal Plus app – which is powered by Welldoc BlueStar and distinct from OneTouch Reveal – also has a bolus calculator. It’s nice to see LifeScan’s continued investment in improving its digital offerings since it was acquired from J&J by private equity firm Platinum Equity in 2018. The other highlight in the booth was the OneTouch Verio Reflect, which, as of the last update in February at ATTD, was available in Canada, France, and Germany. Verio Reflect includes a “Blood Sugar Mentor” that provides on-meter patterns, insights, and encouragement to the user. We like the “Mentor” focused branding, particularly the implication that it will teach and empower the user to be better equipped to manage diabetes in the future.

Insulet
Insulet made its second ever EASD Exhibit Hall appearance, after taking over Omnipod distribution from Ypsomed in 2018. One very engaged US representative was on hand to discuss the newly launched Dash PDM, which has not been launched in Europe. According to the rep, Omnipod Dash has seen very positive feedback from both patients and doctors, and the company is continuing to work hard at expanding US coverage. As of 2Q19, Omnipod Dash was expected for a launch in 2020 in Europe. The new PDM is currently covered for about half of US lives and quickly expanding. We think the no-upfront-cost, pay-as-you-go model for Omnipod Dash is a much easier sell to both payers and consumers, and the pharmacy distribution simplifies startup hassle for everyone.
Medtronic
The main attraction in Medtronic’s booth was the new Envision Pro professional CGM (fully disposable, no-fingerstick-calibration, blinded, seven-day-wear, Bluetooth). See our Day #2 coverage for pictures and more details on Envision Pro.
-
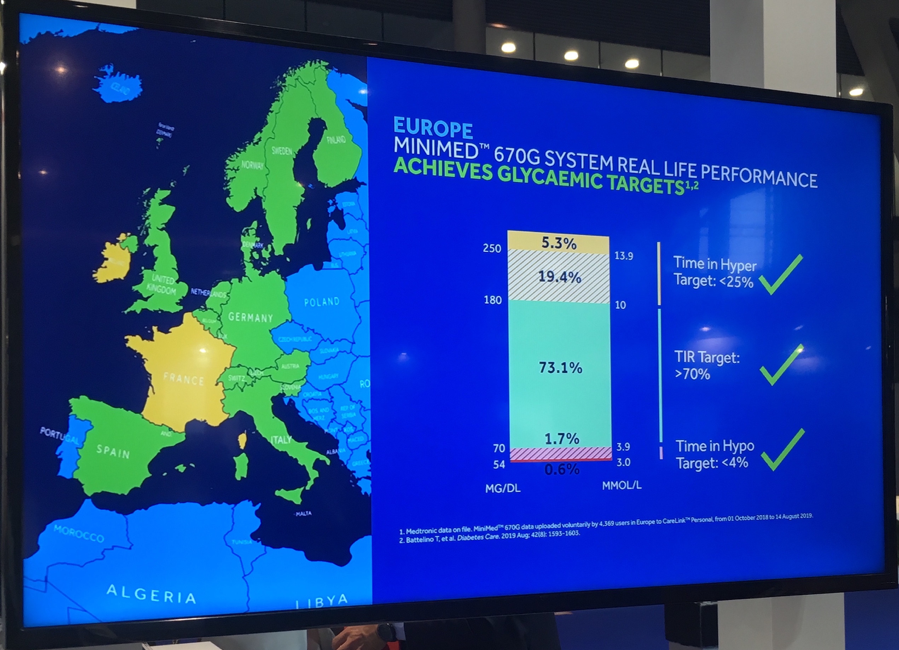
Aside from the new professional CGM, we were glad to see new CareLink data from 4,369 European 670G users – Medtronic also published a press release about this data today. Glycemic outcomes on the system – at least among those who upload data to CareLink – are similar to those seen in the US, headlined by 73% in 70-180 mg/dl and just 2% <70 mg/dl. The release confirmed that there are now >200,000 670G users in 17 countries globally, ~10,000 of which are in Europe (i.e., 95% of global users are in the US). As a reminder, after securing CE Mark in June 2018, 670G began rolling out OUS in October 2018.
Novo Nordisk
Tucked away in Novo Nordisk’s booth, we got our first look at a prototype, Bluetooth-enabled pen attachment for prefilled, disposable insulin pens (highlighted in the image below; red box added by us for emphasis). A rep referred to the attachment as “Dialogue.” The device, which is not yet CE-Marked, sits on the back end of the pen (i.e., on top of the dial) where it can detect the type of insulin that is being delivered by the color of exposed plunger. It has no on-device display, meaning all data viewing will take place in the smartphone app (or potentially a lock-screen widget?). The user essentially treats the device as if it were a part of the pen, twisting to load a dose and pushing down on the plunger to inject (doing so mechanically causes the same to happen on the underlying pen interface). To send the dose to an app via Bluetooth – we saw a Diasend/Glooko demo, though Abbott, Roche, Dexcom, and Medtronic will certainly take in the data via existing partnerships – one simply presses the plunger a second time. The device has an impressive 1,200-injection memory and a two-year battery life (compare to the one-year life of Companion Medical’s InPen and the need to recharge Common Sensing’s GoCap). We’re not sure how it stacks up on features to Lilly’s under-FDA-review smart pen, on which few details have been released.
-
The booth was also showing off the company’s CE-Marked, NFC-enabled, reusable pens (Novo Pen 6 and Echo Plus). A rep confirmed timing that both the NFC- and Bluetooth-enabled devices will launch in 2020 – back from initial guidance for early 2019 – with the Bluetooth attachment for disposable pens following the NFC-enabled durable pens. We continue to assume a Europe-first launch, with the US to follow. A rep told us that there are no near-term plans to shift the NFC durable connected pens to Bluetooth – users will have to scan the pen with their phones or other uploaders in order to bring the data into an app. (This is quite an interim step, but better than no smart pen data at all!) Particularly given Novo Nordisk’s slate of data partners, which includes all three major CGM manufacturers, Roche, and Glooko, we are eager to see these devices advance to market and begin filling the critical gap in clinical care that is access to insulin injection history. Two posters at ADA described the positive impact on time-in-range and as a result of the introduction of the NFC pens into select Swedish clinics.

Roche
Roche’s booth was all about showing off the Accu-Chek Solo patch pump. Initially in pilot across ~200 patients in the UK, Austria, Switzerland, and Poland, representatives confirmed that the pump is currently being expanded to other markets in Europe and the Middle East. This product has scaled quite slowly and we’ll be interested to see how much investment it gets. Reps also hinted at a planned US launch of the pump at some point in the future, though we imagine that could be far off at the current pace. We were also given an active demonstration of the “Meter-Free BGM” via Smartphone Camera + Strips (Accu-Chek Sugar View) for non-insulin-using type 2 diabetes. Roche is targeting this product in emerging markets where access to healthcare is limited. It has expanded its pilot to India and Pakistan (as of July, it was only in Nigeria, Mexico, and the Philippines). Users gain access to the mobile application free of charge and only need to purchase test strips. With two simple scans of the strips, patients can see their blood glucose levels within a range (e.g., 70-130 mg/dl or 131-180 mg/dl) within 45 seconds and view results.
SOOIL
SOOIL showed off DANA Diabecare-i, it’s latest in-development Bluetooth pump. The pump is (and looks) very similar to the DANA RS, differing in that it uses AAA batteries instead of AA, it is waterproof, and has other small user interface improvements (e.g., a bigger window to allow for easier viewing of the reservoir). Like the RS, the i has apps for both Android and iOS, as well as pump control from smartphone. Reps said that the i has been submitted to FDA as an ACE pump and positioned FDA clearance as expected in 2020 – that could make SOOIL the second ACE Pump following Tandem’s t:slim X2. A CE Mark is expected in a “similar time frame, but maybe later.” On the automated insulin delivery side, reps said that SOOIL does have an algorithm partner (not disclosed), but has not settled on a CGM partner just yet. DANA pumps are especially popular in the international DIY community, and it will be interesting to see how it is perceived in the US where Omnipod (non-Dash) and legacy Medtronic pumps are already compatible for DIY, and the AID competitive landscape will be more filled out (Tandem Control-IQ, Medtronic 780G, possibly Omnipod Horizon and Tidepool Loop) by the time DANA i comes to market.
The diaTribe Foundation’s Sixth Annual Solvable Problems in Diabetes Forum
1. The diaTribe Foundation’s “Solvable Problems in Diabetes” Brings Drs. Mathieu, Amiel, and Chan, and Vilsbøll Together to Discuss EASD 2019, New ESC Guidelines, Diabetes Advocacy, and More
On Tuesday night, the diaTribe Foundation hosted the 6th Annual Solvable Problems in diabetes, putting together a fascinating panel featuring Profs. Chantal Mathieu, Juliana Chen, Stephanie Amiel, and Tina Vilsbøll. Below, we’ve provided a number of quotable quotes to capture the many incredible insights shared.
On what to most look forward to at EASD 2019
-
“My main interest is understanding and preventing hypoglycemia, particularly in type 1 diabetes. I’m also interested not so much in technology development, which is fantastic and definitely happening quickly, in also understanding how people use the technology. We have the assumption that technologies will solve problems and while they can, we’re rushing forward with them without always understanding everything we could. One of the things we have to understand is how people work with these technologies.” – Dr. Amiel
-
“I look forward to seeing very interesting implementation science in order to make sure people who need the latest drugs and technology get it … A lot has been going on over the last 20-30 years, and the question is now how do we use it [technology] and at what time. Giving the right drug, the right technology, to the right people, for the right reason.” – Dr. Chan
-
“I’m a type 2 diabetologist. Looking back 21 years ago, at my first conference, it wasn’t fun being a type 2 diabetologist. Everyone was talking about type 1 diabetes. Being a type 2 diabetologist is actually really, really fun this year.. And from scientific point of view, I’m indeed looking forward to future treatments for obesity with and without diabetes coming up. Obesity today is where diabetes was 20 years ago. To have new and better treatments for obesity is of major importance for the future.” – Dr. Vilsbøll
-
“As VP of the EASD, of course all the sessions are interesting – we do our best. What is really interesting me the most is finding new biomarkers, and this concept of heterogeneity. Heterogeneity in type 1, also in type 2, and also in therapeutic response. I’m always envious of the cancer field and where they are in phenotyping and genotyping individuals and giving them the right therapies, avoiding futile therapies, improving outcomes. Scientifically that’s the big goal for me.” – Dr. Mathieu
-
“We should also admit that we’ve been living in amazing times for the past 15 years, especially in type 2 diabetes. I don’t know if progress will be SO huge in the next five years. Of course I hope it will be, but the bar for new therapies – given SGLT2 and GLPP1 hard outcomes – will be very high. I see pharma companies turning away from diabetes and looking at obesity and the disease apparently we’ll all die from, NASH. I’m just saying that things have been going fast and it may not always be this way.” – Dr. Mathieu
On individualization of care and improving population level outcomes
-
“Everyone with diabetes is unique: young, old, lean, obese. Tons of risk factors, tons of complications. But not until you systematically profile people can you individualize care. 20 odd years ago we were seeing so many patients and they would have renal disease without ever having had their kidneys checked in the past 6 years. We need more structured care. A team, and a system for monitoring. They can have a risk profile (eye, feet, blood, urine) and that gives you 20 or 30 variables, which already gives you several combinations of risk, and from there you can start individualizing care. The information is important. We need this data to empower and engage these people with their own risk, and then give them informed choice. Finally, there is this ongoing engagement with the patient and a continuing relationship. Everyone knows about team-based care… you need lay people to help us. Peers, supporters. But you still need environment too. But they come here, they learn about themselves, they learn about the latest technology. We need to document what we do, and decide what kind of care” – Dr. Chan
-
“The voice of the person living with diabetes, that’s what works. That’s how we got Libre, that’s how we get things reimbursed. Bringing them to look payers in the eyes and say we need to do this. We can show all the evidence in the world, but it is their voices that need to say it.” – Dr. Mathieu
On the new ESC/EASD Guidelines (see ESC Days #1-3 Highlights)
-
“This is about, if you’re not on metformin, go straight to SGLT-2 or GLP-1 if you have CVD on diagnosis. That was not good for EASD, because if you look at all of our big intervention trials, you do have metformin in the background in >80% of the people. That was an issue to us at EASD. There will be an ADA/EASD consensus update. We do acknowledge that beneficial effects of SGLT-2/GLP-1s seems to be independent of A1c effect.” – Dr. Mathieu
-
“I hate to be a naysayer, but these aren’t medications entirely without side effects for some people. The diabetes community is more familiar with these drugs, but the cardiologists have a different way of thinking. You see things happening very beautifully in trials where everything is very well controlled and we wish everyone could be in a trial because outcomes are better in trials even in the placebo group. But you have to be aware that you’re treating a much wider section of the community than the population in trials. If things go wrong, we need to be in a position to fix them up quickly. I think it is very exciting, and I think we should be moving much more quickly than we are – but I just want to add a note of caution. We are poised to improve outcomes substantially, but we need to do it carefully.” – Dr. Amiel
-
“I think it’s a bit too early, but I love this discussion. Cardiologists were very quick, but this is a discussion we need. Of course, the cardiologists are overwhelmed, because it’s the first time they have an efficient drug for HF for a very long time – we gave them a gift! In the UK, I know that even nurses may prescribe heart medications. Soon, they might be prescribing SGLT-2-inhibitors and GLP-1s to patients with pre-diabetes or even no diabetes. I’m afraid this will mask the diabetes and they [may not] see an endocrinologist. I’m sure the cardiologists won’t check the feet, won’t talk about ED since they seldomly have interest for what is below the diaphragm, etc.” – Dr. Vilsbøll
On Diabetes Advocacy
-
“The voice of the person living with diabetes, that’s what works. That’s how we got Libre, and other things reimbursed. By bringing them to look payers in the eyes and say we need to do this. We can show all the evidence in the world, but it is their voices that need to say it. Our payers start to look at the ceiling when people with diabetes walk in because they think ‘Oh my god, they will get it!’ All other diseases are envious.” – Dr. Mathieu
-
“I think type 1 community is very vocal and works well (with advocacy) often. The type 2 community is much more diffuse, and in many parts of the world a big issue is stigma. Trying to get people to come forward for treatment, and to advocate for future treatment, can be very difficult. One thing we have to deal with is stigma for obesity rolling over to people with diabetes. There’s much more research funding for CVD and cancer. Public perception is that diabetes is diabetes, with no distinction (between type 1 and 2). It can be difficult to get enthusiasm amongst communities, politicians, until you can get through the stigma that currently attaches obesity and through obesity to diabetes.” – Dr. Amiel
-
“I believe in data. Facts and figures are very persuasive. How can we systematically collect this data and track them over time? We’re in a big data world – especially with CGM and these new medications. We must combine this data with research and engage the practitioners and managers so they are all together, trying to learn from the patient. In Hong Kong that’s what we did. Twenty years ago, we were short of staff and had no choice but to use a lot of nurses and healthcare assistants so we structured the program so they could do a lot. Learning a lot from the protocol of a clinical trial – that seems to work.” – Dr. Chan
On Tele-medicine
-
“We’ve talked about places where 80% of people are using technology, but my clinic in South London isn’t like that, unfortunately. Sometimes, an education group is the first time people get to talk to someone else with type 1. To see people who have felt terribly isolated with a disease they don’t want to talk about, interacting with each other, is transformative. Our new programs are trying to mix together technology and face-to-face interaction. Yes, it’s more time consuming, but the benefits are much greater, because people learn from people.” – Dr. Amiel
-
“In 10 or 20 years, the doctors will be very familiar with technology, and on top of the technology getting better. Maybe you’ll only need to see the patient once a year, with technology in-between, but I don’t think you’ll ever replace the face-to-face interaction. I gave an e-learning talk with 20 patients and it was very good. That kind of group learning can be very empowering, and in a setting like that they don’t have to travel.” – Dr. Chan
--by Albert Cai, Anirudh Gururaj, Ursula Biba, Rhea Teng, Abigail Dove, Brian Levine, Martin Kurian, and Kelly Close