Pharnext raises € 7.7 million in a private placement
This information is not intended for, and shall not be accessible, published, distributed or circulated to persons resident or located in the United States of America, Canada, Japan or Australia, and is not an offer to subscribe for or sell, nor a solicitation of an offer to subscribe for or buy securities of Pharnext in the United States, Canada, Japan or Australia. Any person who wishes to access the information and documents contained on this website must first satisfy himself that he is not subject to local laws or regulations prohibiting or restricting such right of access or requiring registration or approval of the securities in order to acquire them. Pharnext will not accept any liability arising out of the breach by any person of the applicable laws or regulations. By scrolling down, I confirm that I have read and agree to these terms and undertake to comply with applicable regulations.
NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES,
CANADA, AUSTRALIA OR JAPAN
PARIS, France, 08:30 a.m., March 05, 2020 (CET) – Pharnext SA (FR0011191287 - ALPHA) (the “Company”), a biopharmaceutical company pioneering a new approach to developing innovative drug combinations based on its PLEOTHERAPY artificial intelligence platform harnessing big genomics data and network pharmacology, today announced a capital raise of circa € 7.7 million by way of issuance of 1,799,061 new ordinary shares (the “New Shares”) with one warrant attached each (together with the New Shares, the “ABSA”). The capital raise was led by existing investors and included several new US-based institutional investors as well as members of Pharnext management, including the Company’s Chief Executive Officer and Chief Financial Officer (the “Capital Raise”).
“We are pleased to announce this capital raise, which demonstrates the strong, continued support of our historical shareholders,” said Peter Collum, Chief Financial Officer and Chief Business Officer of Pharnext. “This financing also helps broaden our institutional shareholder base, and begins to enhance our visibility in the U.S. market.”
This Capital Raise did not and will not require the publication of a prospectus subject to the approval of the Autorité des marchés financiers (“AMF”).
Use of Proceeds
The proceeds of the Capital Raise will provide the Company with the resources to fund operations during the next twelve months, and primarily to finalize the Phase 3 study protocol and other regulatory requirements needed for continuing development of PXT3003 in the treatment of Charcot-Marie-Tooth disease type 1A with the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA).
Terms of the Capital Raise
The ABSA have been placed, without shareholders’ preferential subscription rights, by means of (i) a private placement to qualified investors and a restricted circle of investors pursuant to the 21st resolution of the combined ordinary and extraordinary general meeting of the shareholders of the Company held on June 26th, 2019 (the “General Meeting”), as well as (ii) a share capital raise to investors fitting in the category defined by the 23rd resolution of the General Meeting. The Capital Raise has been decided by the Company’s board of directors on March 04, 2020, in accordance with both 21rd and 23rd resolutions of the General Meeting and with Articles L.225-136 and L. 225-138 of the French Commercial Code (code de commerce).
The issue price of one ABSA is € 4.28, issue premium included, representing a 12.30 % facial discount to the volume weighted-average price of the Company’s shares over the last three trading days (the “3-day VWAP”) i.e € 4.88. The issue price of an ABSA, including the theoretical value of a warrant (“BSA”), represents a total 29.92% discount to the 3-day VWAP, consistent with the 21st and 23rd resolutions of the General Meeting.
Terms of the BSA
One BSA is attached to each New Share.
Four BSAs entitle their holders to subscribe to three new ordinary shares of the Company, at a price of € 5.56 per ordinary share.
The BSAs may be exercised at any time within 42 months of their issuance. In the event all BSAs are exercised a total number of 1,349,298 additional ordinary shares of the Company will be issued, representing an additional total amount of proceeds of approximately € 7.5 million.
The theoretical value of each BSA, assuming a volatility of 45% and based on closing price as of March 04, 2020, is equal to € 0.86 under Black & Scholes model, representing 17.62 % of the 3-day VWAP .
The BSAs will be immediately detached (détachés) from the New Shares upon issuance. The BSAs will not be listed.
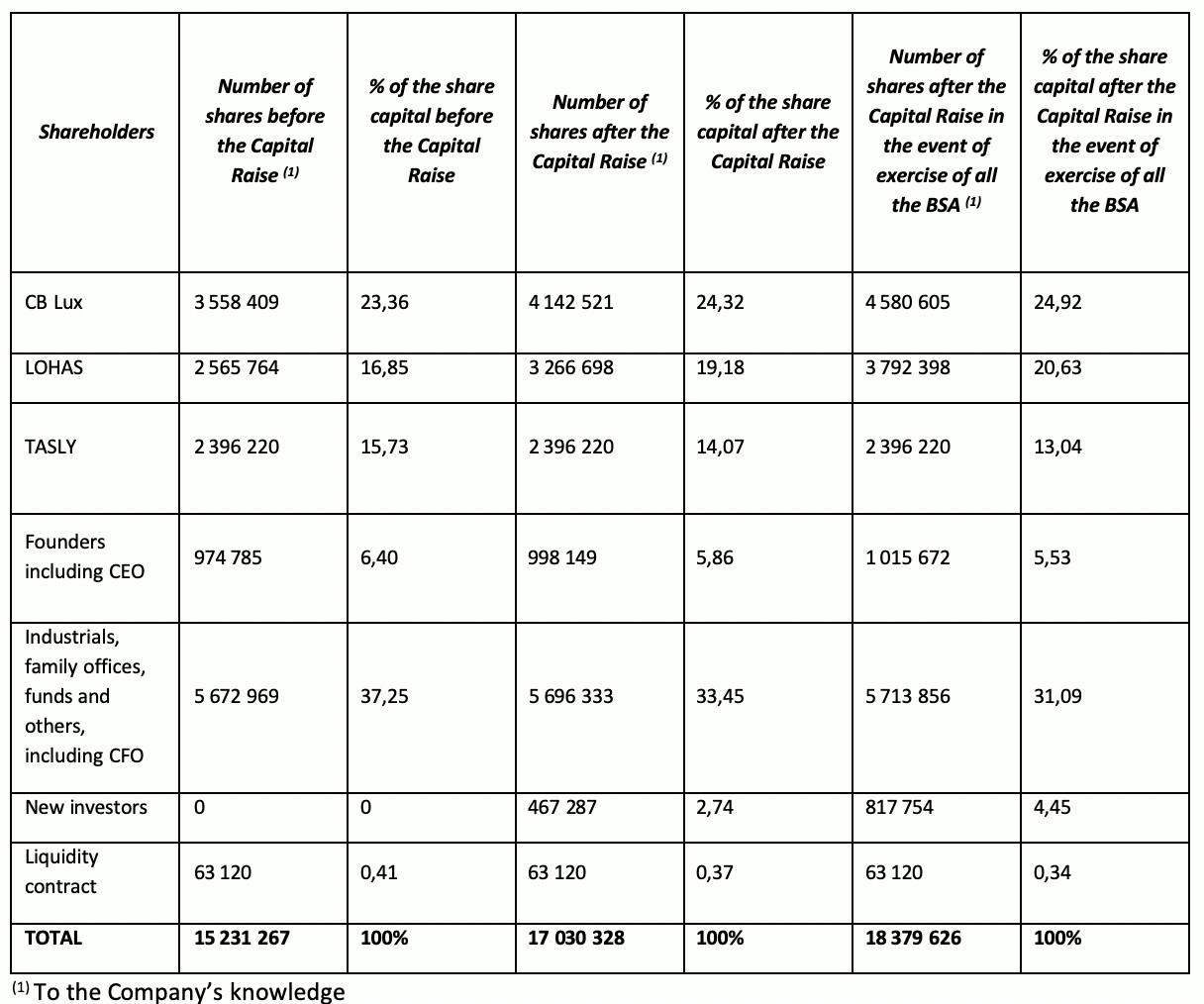
The Company’s shareholding structure after the Capital Raise
Following the issuance of the ABSA, the Company’s total share capital will be € 170,303.28, equal to 17,030,328 ordinary shares (or € 183,796.26, equal to 18,379,626 ordinary shares in the event of exercise of all the BSA) with a par value of €0.01, representing 111.81% of the total current share capital of the Company (or 120.67% in the event of exercise of all the BSA).
Lohas S.à.r.l, a company controlled by Mr. Pierre Bastid, himself a member of the board of directors of the Company, and CB LUX S.à.r.l, holding respectively 16.85% and 23.36% of Pharnext’s share capital prior to the Capital Raise, have subscribed for an aggregate amount of €5,500,000 in the Capital Raise. Following completion of the Capital Raise, they will own 19.18% and 24.32% respectively of the share capital of the Company.
Daniel Cohen, the Chief Executive Officer of the Company, and Peter Collum, the Chief Financial Officer of the Company have subscribed for an aggregate amount of €200,000 in the Capital Raise. Following completion of the Capital Raise, they will own 3.77% and 0.14% respectively of the share capital of the Company.
On an illustrative basis, a shareholder holding 1% of the Company’s share capital before the Capital Raise and who did not participate in the Capital Raise will hold 0.89% of the Company’s shares after the Capital Raise and 0.83% if all the BSA are exercised.
Delivery and listing of the New Shares
The New Shares will be admitted to trading on Euronext Growth upon their settlement and delivery, which is expected to occur on or about March 11, 2020. They will be listed under the same code as the Company’s existing ordinary shares (ISIN: FR0011191287), carry dividend rights as January 1st, 2020 and be immediately fungible in all respects with the Company’s existing shares.
Standstill and lock-up provisions
In connection with the Capital Raise, the Company has entered into a standstill agreement, which restricts the issuance of additional ordinary shares ending 2 weeks after settlement and delivery of the New Shares, subject to customary exceptions as well as the ability to complete a public offering and request a waiver from the Placement Agent. The Company’s Chief Executive Officer, Chief Financial Officer and board members and supervisory board have also agreed to a 90-day lock-up, subject to the ability to request a waiver from the Placement Agent.
Risk Factors
The Company draws the public’s attention to the risk factors related to the Company and its activities presented in the registration document (document de référence) filed with the AMF on June 2, 2016 under number I.016-050, as well as in its annual periodic management reports and press releases, copies of which are available free of charge on the websites of the Company (www.pharnext.com) and/or the AMF (www.amf-france.org).
About Pharnext
Pharnext is an advanced clinical-stage biopharmaceutical company developing novel therapeutics for orphan and common neurodegenerative diseases that currently lack curative and/or disease-modifying treatments. Pharnext has two lead products in clinical development. PXT3003 completed an international Phase 3 trial with positive topline results for the treatment of Charcot-Marie-Tooth disease type 1A and benefits from orphan drug status in Europe and the United States. PXT864 has generated encouraging Phase 2 results in Alzheimer’s disease. Pharnext has developed a new drug discovery paradigm based on big genomics data and artificial intelligence: PLEOTHERAPY™. Pharnext identifies and develops synergic combinations of drugs called PLEODRUG™. The Company was founded by renowned scientists and entrepreneurs including Professor Daniel Cohen, a pioneer in modern genomics, and is supported by a world-class scientific team. More information at www.pharnext.com.
Pharnext is listed on the Euronext Growth Stock Exchange in Paris (ISIN code: FR0011191287).
Disclaimer:
In accordance with Article L. 411-2 of the French Monetary and Financial Code and applicable regulations, no prospectus has been or will be published or submitted for approval to the AMF in connection with the above placement.
With respect to the Member States of the European Economic Area, no action has been taken or will be taken to allow an offer to the public of the securities referred to in this press release, requiring the publication of a prospectus (pursuant to article 3 of the Regulation (EU) 2017/1129 of the European Parliament and of the Council of June 14, 2017) in any of the Member States.
This press release and the information it contains is not an offer to subscribe for or sell, nor the solicitation of an offer to subscribe for or buy, securities of Pharnext in the United States or any other jurisdiction. Securities may not be offered or sold in the United States absent registration or an exemption from registration under the U.S. Securities Act of 1933, as amended. Pharnext does not intend to conduct a public offering in the United States or in any other jurisdiction.
This distribution of this press release may be subject to legal or regulatory restrictions in certain jurisdictions. Any person who comes into possession of this press release must inform him or herself of and comply with any such restrictions.


