Long-held to be a post-translational modification unique to Eukarya, it is now clear that Bacteria and Archaea also perform N-glycosylation, the covalent linkage of glycans to select asparagine residues of target proteins. However, whereas such protein processing is restricted to a limited number of bacterial groups, N-glycosylation is an almost universal event in the Archaea. Work in our lab seeks to better understand the archaeal version of this universal post-translational modification using bioinformatics, genetic, biochemical, and structural approaches.
From recent publications:
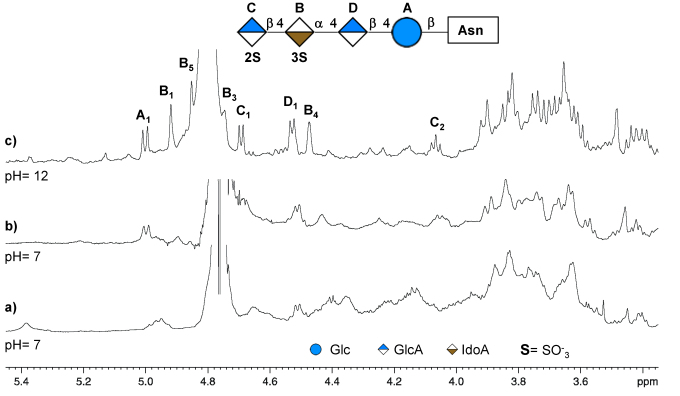
Notaro, A., Vershinin, Z., Guan, Z., Eichler, J. and De Castro, C. (2022) An N-linked tetrasaccharide from Halobacterium salinarum presents a novel modification, sulfation of iduronic acid at the O-3 position. Carbohydr. Res., 521, 108651.
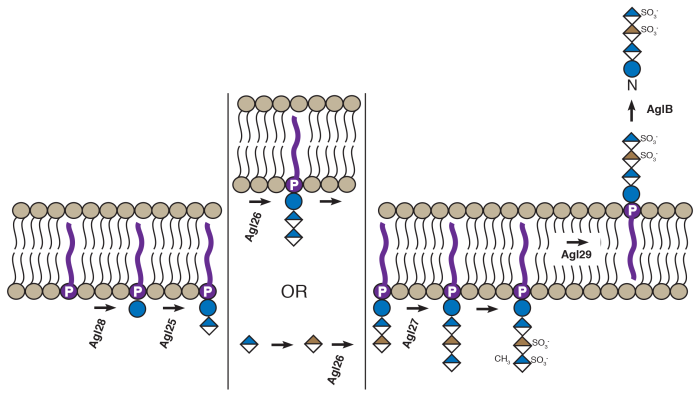
Vershinin, Z., Zaretsky, M., Guan, Z., and Eichler, J. (2023) Agl28 and Agl29 are key components of a Halobacterium salinarum N-glycosylation pathway. FEMS Microbiol. Lett., 370, fnad017.