Abstract
Although cooking is regarded as a key element in the evolutionary success of the genus Homo, impacting various biological and social aspects, when intentional cooking first began remains unknown. The early Middle Pleistocene site of Gesher Benot Ya’aqov, Israel (marine isotope stages 18–20; ~0.78 million years ago), has preserved evidence of hearth-related hominin activities and large numbers of freshwater fish remains (>40,000). A taphonomic study and isotopic analyses revealed significant differences between the characteristics of the fish bone assemblages recovered in eight sequential archaeological horizons of Area B (Layer II-6 levels 1–7) and natural fish bone assemblages (identified in Area A). Gesher Benot Ya’aqov archaeological horizons II-6 L1–7 exhibited low fish species richness, with a clear preference for two species of large Cyprinidae (Luciobarbus longiceps and Carasobarbus canis) and the almost total absence of fish bones in contrast to the richness of pharyngeal teeth (>95%). Most of the pharyngeal teeth recovered in archaeological horizons II-6 L1–7 were spatially associated with ‘phantom’ hearths (clusters of burnt flint microartifacts). Size–strain analysis using X-ray powder diffraction provided evidence that these teeth had been exposed to low temperature (<500 °C), suggesting, together with the archaeological and taphonomic data, that the fish from the archaeological horizons of Area B had been cooked and consumed on site. This is the earliest evidence of cooking by hominins.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The archaeological and palaeontological materials used in this study are held at the National Natural History Collections of the Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Israel; the fish reference collection and the XRD samples are held at the SMNH, Tel Aviv University; and the teeth sampled for the isotope analysis are held at the Institute of Geosciences, Johannes Gutenberg University of Mainz, Germany. All data are available in the main text or the Supplementary Information.
Change history
20 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41559-023-02270-y
References
Stewart, K. M. Early hominid utilisation of fish resources and implications for seasonality and behaviour. J. Hum. Evol. 27, 229–245 (1994).
Braun, D. R. et al. Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proc. Natl Acad. Sci. USA 107, 10002–10007 (2010).
Steele, T. E. A unique hominin menu dated to 1.95 million years ago. Proc. Natl Acad. Sci. USA 107, 10771 (2010).
Lipato, I. & Kapute, F. Nutritional quality of Barbus paludinosus (matemba) smoked using traditional and improved smoking methods. Int. Food Res. J. 24, 1507–1512 (2017).
Ljubojević, D. et al. Fatty acid composition of fishes from inland waters. Bulg. J. Agric. Sci. 19, 62–71 (2013).
Cunnane, S. & Stewart, K. M. Human Brain Evolution (John Wiley & Sons, 2010).
Broadhurst, C. L., Cunnane, S. C. & Crawford, M. A. Rift Valley lake fish and shellfish provided brain-specific nutrition for early Homo. Br. J. Nutr. 79, 3–21 (1998).
Panth, N., Gavarkovs, A., Tamez, M. & Mattei, J. The influence of diet on fertility and the implications for public health nutrition in the United States. Front. Public Health 6, 211–211 (2018).
Bastías, J. M., Balladares, P., Acuña, S., Quevedo, R. & Muñoz, O. Determining the effect of different cooking methods on the nutritional composition of salmon (Salmo salar) and Chilean jack mackerel (Trachurus murphyi) fillets. PLoS ONE 12, e0180993 (2017).
Choo, P., Azlan, A. & Khoo, H. E. Cooking methods affect total fatty acid composition and retention of DHA and EPA in selected fish fillets. Sci. Asia 44, 92–101 (2018).
Berna, F. et al. Microstratigraphic evidence of in situ fire in the Acheulean strata of Wonderwerk Cave, Northern Cape province, South Africa. Proc. Natl Acad. Sci. USA 109, E1215–E1220 (2012).
Wrangham, R. Control of fire in the Paleolithic: evaluating the cooking hypothesis. Curr. Anthropol. 58, S303–S313 (2017).
Chazan, M. et al. Renewed excavations at Wonderwerk Cave, South Africa. Evol. Anthropol. 26, 258–260 (2017).
Wadley, L. et al. Fire and grass-bedding construction 200 thousand years ago at Border Cave, South Africa. Science 369, 863–866 (2020).
Hlubik, S., Berna, F., Feibel, C., Braun, D. & Harris, W. K. J. Researching the nature of fire at 1.5 Ma on the site of FxJj20 AB, Koobi Fora, Kenya, using high-resolution spatial analysis and FTIR spectrometry. Curr. Anthropol. 58, S243–S257 (2017).
Withnell, C. & de la Torre, I. Thermal alterations in experimentally-flaked stone tools from Olduvai Gorge and their relevance for identification of fire in the Early Stone Age. J. Archaeol. Sci. Rep. 27, 101978 (2019).
MacDonald, K., Scherjon, F., van Veen, E., Vaesen, K. & Roebroeks, W. Middle Pleistocene fire use: the first signal of widespread cultural diffusion in human evolution. Proc. Natl Acad. Sci. USA 118, e2101108118 (2021).
Larbey, C., Mentzer, S. M., Ligouis, B., Wurz, S. & Jones, M. K. Cooked starchy food in hearths ca. 120 kya and 65 kya (MIS 5e and MIS 4) from Klasies River Cave, South Africa. J. Hum. Evol. 131, 210–227 (2019).
Wadley, L., Backwell, L., d’Errico, F. & Sievers, C. Cooked starchy rhizomes in Africa 170 thousand years ago. Science 367, 87–91 (2020).
Stiner, M. C., Kuhn, S. L., Weiner, S. & Bar-Yosef, O. Differential burning, recrystallisation, and fragmentation of archaeological bone. J. Archaeol. Sci. 22, 223–237 (1995).
Shipman, P., Foster, G. & Schoeninger, M. Burnt bones and teeth: an experimental study of color, morphology, crystal structure and shrinkage. J. Archaeol. Sci. 11, 307–325 (1984).
Lebon, M., Reiche, I., Fröhlich, F., Bahain, J. J. & Falguères, C. Characterization of archaeological burnt bones: contribution of a new analytical protocol based on derivative FTIR spectroscopy and curve fitting of the nu1nu3 PO4 domain. Anal. Bioanal. Chem. 392, 1479–1488 (2008).
Hlubik, S. et al. Hominin fire use in the Okote member at Koobi Fora, Kenya: new evidence for the old debate. J. Hum. Evol. 133, 214–229 (2019).
Goren-Inbar, N., Alperson-Afil, N., Sharon, G. & Herzlinger, G. The Acheulian site of Gesher Benot Ya’aqov (Springer International Publishing, 2018).
Rabinovich, R. & Biton, R. The Early–Middle Pleistocene faunal assemblages of Gesher Benot Ya’aqov: inter-site variability. J. Hum. Evol. 60, 357–374 (2011).
Alperson-Afil, N. et al. Spatial organization of hominin activities at Gesher Benot Ya’aqov, Israel. Science 326, 1677–1680 (2009).
Zohar, I., Goren, M. & Goren-Inbar, N. Fish and ancient lakes in the Dead Sea Rift: the use of fish remains to reconstruct the ichthyofauna of palaeo-lake Hula. Palaeogeogr. Palaeoclimatol. Palaeoecol. 405, 28–41 (2014).
Goren-Inbar, N., Melamed, Y., Zohar, I., Akhilesh, K. & Pappu, S. Beneath still waters—multistage aquatic exploitation of Euryale ferox (Salisb.) during the Acheulian. Internet Archaeol. https://doi.org/10.11141/ia.37.1 (2014).
Melamed, Y., Kislev, M. E., Geffen, E., Lev-Yadun, S. & Goren-Inbar, N. The plant component of an Acheulian diet at Gesher Benot Ya’aqov, Israel. Proc. Natl Acad. Sci. USA 113, 14674–14679 (2016).
Goren, M. & Ortal, R. Biogeography, diversity and conservation of the inland water fish communities in Israel. Biol. Conserv. 89, 1–9 (1999).
Zohar, I. & Biton, R. Land, lake, and fish: investigation of fish remains from Gesher Benot Ya’aqov (palaeo-lake Hula). J. Hum. Evol. 60, 343–356 (2011).
Ljubojević, D., Đorđević, V. & Ćirković, M. Evaluation of nutritive quality of common carp, Cyprinus carpio L. IOP Conf. Ser. Earth Environ. Sci. 85, 012013 (2017).
Lindy, M. B. & Chapman, L. J. Foraging costs of hypoxia acclimation in the swamp-dwelling African Cyprinid, Barbus neumayeri. Copeia 2006, 552–557 (2006).
Alperson-Afil, N. & Goren-Inbar, N. The Acheulian Site of Gesher Benot Ya’aqov: Ancient Flames and Controlled Use of Fire (Springer, 2010).
Denys, C. et al. Biominerals fossilisation: fish bone diagenesis in Plio–Pleistocene African hominid sites of Malawi. Minerals 10, 1049–1069 (2020).
Guillaud, E., Bearez, P., Denys, C. & Raimond, S. New data on fish diet and bone digestion of the Eurasian otter (Lutra lutra) (Mammalia: Mustelidae) in central France. Eur. Zool. J. 84, 226–237 (2017).
Vasilyan, D., Roček, Z., Ayvazyan, A. & Claessens, L. Fish, amphibian and reptilian faunas from latest Oligocene to middle Miocene localities from Central Turkey. Palaeobio. Palaeoenv. 99, 723–757 (2019).
Villagran, X. S. A redefinition of waste: deconstructing shell and fish mound formation among coastal groups of southern Brazil. J. Anthropol. Archaeol. 36, 211–227 (2014).
Nurminen, K. Taphonomy of burned fish bones—burning experiments in the open fire. Environ. Archaeol. 21, 157–160 (2016).
Wilson, M. V. H. Predation as a source of fish fossils in Eocene lake sediments. Palaios 2, 497–504 (1987).
Shimosaka, C., Shimomura, M. & Terai, M. Changes in the physical properties and composition of fish bone during cooking by heating under normal pressure. J. Home Econ. Jpn 47, 1213–1218 (1996).
Richter, J. Experimental study of heat-induced morphological changes in fish bone collagen. J. Archaeol. Sci. 13, 477–481 (1986).
Ishikawa, M., Mori, S., Watanabe, H. & Sakai, Y. Softening of fish bone. II. Effect of acetic acid on softening rate and solubilization rate of organic matter from fish bone. J. Food Process. Preserv. 13, 123–132 (1989).
Zohar, I., Ovadia, A. & Goren-Inbar, N. The cooked and the raw: a taphonomic study of cooked and burned fish. J. Archaeol. Sci. Rep. 8, 164–172 (2016).
Analytic Rarefaction 1.3 (UGA Stratigraphic Lab, 2003); https://strata.uga.edu/software/anRareReadme.html
Krebs, C. J. Ecological Methodology 2nd edn (Harper Collins Publishers, 1999).
Feibel, C. S. in Human Paleoecology in the Levantine Corridor (eds Goren-Inbar, N. & Speth, J. D.) 21–36 (Oxbow Books, 2004).
Feibel, C. S. in Sediments in Archaeological Context (eds Stein, J. K. & Farrand, W. R.) 127–148 (The Univ. of Utah Press, 2001).
Thoms, A. V., Short, L. M., Kamiya, M. & Laurence, A. R. Ethnographies and actualistic cooking experiments: ethnoarchaeological pathways toward understanding earth-oven variability in archaeological records. Ethnoarchaeology 10, 76–98 (2018).
Piga, G. et al. Understanding the crystallinity indices behavior of burned bones and teeth by ATR-IR and XRD in the presence of bioapatite mixed with other phosphate and carbonate phases. Int. J. Spectrosc. 2016, 4810149 (2016).
Piga, G. et al. A multi-technique approach by XRD, XRF, FT-IR to characterize the diagenesis of dinosaur bones from Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 310, 92–107 (2011).
Piga, G., Thompson, T. J. U., Malgosa, A. & Enzo, S. The potential of X-ray diffraction in the analysis of burned remains from forensic contexts. J. Forensic Sci. 54, 534–539 (2009).
Koch, P. L., Tuross, N. & Fogel, M. L. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J. Archaeol. Sci. 24, 417–429 (1997).
LeGeros, R. Z., Trautz, O. R., Legeros, J. P., Klein, E. & Shirra, W. P. Apatite crystallites: effects of carbonate on morphology. Science 155, 1409–1411 (1967).
Pollard, A., Batt, C. M., Stern, B. & Young, S. M. M. Analytical Chemistry in Archaeology (Cambridge Univ. Press, 2007).
Miake, Y. et al. Ultrastructural studies on crystal growth of enameloid minerals in elasmobranch and teleost fish. Calcif. Tissue Int. 48, 204–217 (1991).
Davis, M., Matmon, A., Fink, D., Ron, H. & Niedermann, S. Dating Pliocene lacustrine sediments in the central Jordan Valley, Israel—implications for cosmogenic burial dating. Earth Planet. Sci. Lett. 305, 317–327 (2011).
Alperson-Afil, N., Richter, D. & Goren-Inbar, N. Evaluating the intensity of fire at the Acheulian site of Gesher Benot Ya’aqov—spatial and thermoluminescence analyses. PLoS ONE 12, e0188091 (2017).
Sisma-Ventura, G. et al. Past aquatic environments in the Levant inferred from stable isotope compositions of carbonate and phosphate in fish teeth. PLoS ONE 14, e0220390 (2019).
Pellegrini, M., Lee-Thorp, J. A. & Donahue, R. E. Exploring the variation of the δ18Op and δ18Oc relationship in enamel increments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 310, 71–83 (2011).
Spiro, B. et al. Climate variability in the Upper Jordan Valley around 0.78 Ma, inferences from time-series stable isotopes of Viviparidae, supported by mollusc and plant palaeoecology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 282, 32–44 (2009).
Stiller, M. & Magaritz, M. Carbon-13 enriched carbonate in interstitial waters of Lake Kinneret sediments. Limnol. Oceanogr. 19, 849–853 (1974).
Horowitz, A. The Quaternary Evolution of The Jordan Valley (Dr W. Junk Publishers, 1978).
Horowitz, A. The Jordan Rift Valley (Swets & Zeitlinger B.V. Lisse, 2001).
Zohar, I. et al. The living and the dead: how do taphonomic processes modify relative abundance and skeletal completeness of freshwater fish. Palaeogeogr. Palaeoclimatol. Palaeoecol. 258, 292–316 (2008).
Wrangham, R. Catching Fire: How Cooking Made Us Human (Basic Books, 2009).
Wrangham, R. & Carmody, R. Human adaptation to the control of fire. Evol. Anthropol. 19, 187–199 (2010).
Henry, A. G. Neanderthal cooking and the costs of fire. Curr. Anthropol. 58, S329–S336 (2017).
Joordens, J. C. A., Wesselingh, F. P., de Vos, J., Vonhof, H. B. & Kroon, D. Relevance of aquatic environments for hominins: a case study from Trinil (Java, Indonesia). J. Hum. Evol. 57, 656–671 (2009).
Budka, H. et al. The question of fuel for cooking in ancient Egypt and Sudan. EXARC J. https://exarc.net/ark:/88735/10398 (2019).
Black, S. L. & Thorns, A. V. Hunter-gatherer earth ovens in the archaeological record: fundamental concepts. Am. Antiq. 79, 204–226 (2014).
Stewart, H. Indian Fishing: Early Methods on the Northwest Coast (Univ. of Washington Press, 1982).
Wandsnider, L. The roasted and the boiled: food composition and heat treatment with special emphasis on pit-hearth cooking. J. Anthropol. Archaeol. 16, 1–48 (1997).
Carson, M. T. TĪ ovens in Polynesia: ethnological and archaeological perspectives. J. Polyn. Soc. 111, 339–370 (2002).
Thomas, D. H. On distinguishing natural from cultural bone in archaeological sites. Am. Antiq. 36, 366–371 (1971).
Grayson, D. K. Quantitative Zooarchaeology: Topics in the Analysis of Archaeological Faunas (Academic Press, 1984).
Zohar, I., Dayan, T., Goren, M., Nadel, D. & Hershkovitz, I. Opportunism or aquatic specialization? Evidence of freshwater fish exploitation at Ohalo II—a waterlogged Upper Paleolithic site. PLoS ONE 13, e0198747 (2018).
LeGeros, R. Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 15, 1–201 (1991).
Bar-Yosef, O. & Goren-Inbar, N. The Lthic Assemblages of ‘Ubeidiya (Hebrew Univ. of Jerusalem, 1993).
Dettmann, D. L. et al. Seasonal stable isotope evidence for a strong Asian monsoon throughout the past 10.7 m.y. Geology 29, 31–34 (2001).
Sisma-Ventura, G. et al. Tooth oxygen isotopes reveal Late Bronze Age origin of Mediterranean fish aquaculture and trade. Sci. Rep. 8, 14086–14097 (2018).
Apolinarska, K., Pełechaty, M. & Noskowiak, D. Differences in stable isotope compositions of freshwater snails from surface sediments of two Polish shallow lakes. Limnologica 53, 95–105 (2015).
Dimentman, C., Bromley, H. J. & Por, D. F. Lake Hula: Reconstruction of the Fauna and Hydrobiology of a Lost Lake (The Israel Academy of Sciences and Humanities, 1992).
Marder, O. et al. Jordan River Dureijat: a new epipaleolithic site in the Upper Jordan Valley. J. Israel Prehist. Soc. 45, 5–29 (2015).
Kallaste, T. & Nemliher, J. Apatite varieties in extant and fossil vertebrate mineralized tissues. J. Appl. Crystallogr. 38, 587–594 (2005).
Kallaste, T. & Nemliher, J. Conodont bioapatite resembles vertebrate enamel by XRD properties. Est. J. Earth Sci. 61, 191–192 (2012).
Nemliher, J. & Kallaste, T. Thermal behaviour of bone apatite of recent Pike (Esox lucius L). Proc. Est. Acad. Sci. 54, 112–118 (2005).
Nemliher, J. G., Baturin, G. N., Kallaste, T. E. & Murdmaa, I. O. Transformation of hydroxyapatite of bone phosphate from the ocean bottom during fossilization. Lithol. Miner. Resour. 39, 468–479 (2004).
Acknowledgements
This study was supported by the Israel Science Foundation (ISF) Center of Excellence for the study of ‘Climate change in the Upper Jordan Valley between ca. 800 Ma and 700 Ma ago—its impact on the environment and hominins and its potential as a prediction for future scenarios’ (grant no. 300/06 and grant no. 858/09); ISF grant ‘The nature, scope and interpretation of the Acheulian variability at GBY’ (grant no. 27/12); Irene Levi Sala CARE Archaeological Foundation (grant nos. 178/09, 5/14 and 206/20); and the Dan David Foundation grant for ‘The search and study of modern humans’. Access to the NHM in London was also supported by the SYNTHESYS+ project, with funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement 823827. Isotope analysis was supported by funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant no. 681450). The Israel Antiquities Authority provided the permit for the archaeological excavations (G-101/97). This study was performed at the National Natural History Collections of the Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram; at the Institute of Archaeology of the Hebrew University, Mount Scopus, Jerusalem, Israel; at the SMNH, Tel Aviv University, Israel; at the Department of Archaeology, University of Western Australia, Perth, Australia; and at the NHM in Brussels, Belgium. We thank T. Vennemann (University of Lausanne) and M. Maus (University of Mainz) for performing the phosphate oxygen isotope analysis of the silver phosphate samples. Micro-CT analyses were performed at the Dan David Center for Human Evolution and Biohistory Research and the Shmunis Family Anthropology Institute, Tel Aviv University, Israel. We thank H. May and A. Pokhojaev for their help and support. Part of this study was performed by I.Z. as a visiting researcher at the Department of Archaeology and Anthropology at the University of Western Australia. We thank M. Goren from the SMNH, who provided us with tremendous help and support in the taxonomic study and the collection of freshwater fish; and W. Van Neer (NHM, Brussels; Royal Belgian Institute of Natural Sciences), M. Richter, P. Campbell and O. Crimmen (NHM, UK) for providing access to their laboratories and osteological collections. We thank the late O. Bar-Yosef for providing us with access to the ‘Ubeidiya fish remains, A. Belfer-Cohen for scientific advice, E. Geffen for statistical assistance, K. Stewart for her constructive review and scientific suggestions to improve this manuscript, and also N. Paz and S. Gavrieli for the English editing.
Author information
Authors and Affiliations
Contributions
I.Z., N.G.-I. and N.A.-A. conceived the main conceptual ideas. I.Z. and M.P. performed the taxonomic identification and analyses of GBY fish remains. I.Z. and J.N. designed and performed the XRD laboratory experiments and analysed and interpreted the data. I.Z. and I.H. conducted the micro-CT scans and their interpretations. G.S.-V. and T.T. performed the stable isotope analysis, the isotope data evaluation and interpretation. N.A.-A. performed the spatial analyses. All authors discussed the results and contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Patricia Eichler, Helen Coxall, Christopher Lowery and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
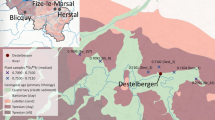
Extended Data Fig. 1 Geographic location of the Gesher Benot Ya’aqov (GBY) site on the paleo shore of Lake Hula (Jordan Rift Valley, Israel).
a, Geographic location of GBY, ‘Ubeidiya and Erq-el-Ahmar (red dots). b, Lake Hula and its surrounding swamps are marked according to their size before the drainage in 1954 (modified from83). Selected archaeological sites (Pleistocene to Holocene) located in the vicinity of palaeo-Lake Hula are denoted by black dots. Rivers and springs draining to Lake Hula appear as blue lines. c, Location of GBY on the modern eastern bank of the Jordan River; main excavated areas appear in coloured squares (Area A = brown, Area B = purple, Area C = orange). d, Archaeological excavation of one of the tilted consecutive archaeological horizons recovered in Area B Layer II-6, with rich material culture. JRD = Jordan River Dureijat84.
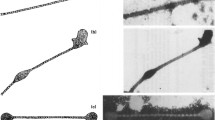
Extended Data Fig. 2 Micro-CT scans of Luciobarbus longiceps (Cyprinidae, Jordan barbel) head, showing the location of the pharyngeal jaws and teeth.
a, Schematic representation of Luciobarbus longiceps (Jordan barbel) elongated body, with two pairs of barbells below the mouth opening. The circle marks the scanned area (TL = total length; HL = head length; Scanned specimen #13.125SMNH). b – c, Micro-CT scans reconstructing the anatomical location of L. longiceps pharyngeal jaw and teeth (fifth ceratobranchials), exhibiting heterodont dentition organized in three rows (teeth formula: 2,3,4-4,3,227). The first molariform tooth (on the 1st row) is adapted for feeding on hard prey, such as mollusks. The teeth are replaced continuously throughout the fish’s life. The enameloid (gold colour) forms only in the penultimate step of differentiation, prior to the attachment of the tooth to the underlying bone, and following complete resorption of the previous functional tooth.
Extended Data Fig. 3 The association between the number (NISP) of fish remains recovered at GBY, by archaeological horizons in three excavated areas (A, B, C) and the level of taxonomic richness and diversity.
a, A summary table showing the total number of fish remains (NISP), the number of remains identified to genus or species level, species richness (S’), and diversity in three excavated areas (A, B, C), and by archaeological horizon. Area A exhibits highest species richness (S’ = 16), while the lowest values for species richness and diversity are observed at the eight sequential AH of Area B II-6 L1–7 (marked in the light blue background; S’ range between 2 to 6 species). b, Rarefaction curves (calculated with PAST) comparing fish remains NISP and species richness identified at GBY, from areas A (red line), B (blue line), and C (green line). On the graph, in each area, the centre line marks the mean change in species richness according to sample size (NISP), whereas the upper and lower lines display the 95% confidence limit of the sample size and species richness. The rarefaction curves show that in areas A, B, and C, fish remains sample size (NISP) did not influence our ability to reconstruct fish population structure (species richness, diversity, and evenness).
Extended Data Fig. 4 Correspondence analyses graphs of excavated areas and fish taxa (a), and of excavated areas and representation of skeletal elements (b).
a, Correspondence analysis graph showing the association between archaeological horizons and fish taxa (Area A; NISP = 9,206, Area B; NISP = 30,318). The two dimensions, the location of the fish remains (51.4%), and the cause of death (natural - Area A, or cultural- Area B; 45%), almost equally account for the data set variation. b, Correspondence analysis graph showing the association between AH and skeletal elements. The deposition agent (natural or cultural) accounts for most of the variation in the data set (80.8%). This derives from the unique preservation pattern observed in the eight superimposed AHs of Area B (II-6 L1–7), that is, comprises almost exclusively cyprinid pharyngeal teeth. Bottom: Representative fish remains recovered at GBY areas A and B (from left to right): Cyprinidae pharyngeal teeth, L. longiceps 1st molariform tooth, C. canis pharyngeal jaw, Clariidae frontal bone, Cyprinidae pectoral spine, and Cichlidae dorsal fin spine.
Extended Data Fig. 5 X-ray diffraction (XRD) analysis of bioapatite lattice parameters, microstrain, and crystallite size (CS) in fish tooth enameloid.
a, Association between lattice parameters ratio (c/a) and estimated stoichiometric coefficient (x) in cyprinid teeth used in this study (n = 49) and in Palaeozoic and Mesozoic (400‒200 Ma) fossil fish teeth (n = 10)85,86,87,88. b, Lattice parameters of cyprinid pharyngeal teeth analysed in this study and in Palaeozoic and Mesozoic fossil fish teeth85,86,87,88. c, Association between enameloid microstrain and CS in: fresh unheated molariform teeth (n = 4), teeth from whole fresh fish experimentally heated to 200‒600 °C (n = 11), fossil cyprinid teeth of GBY (n = 31), and Erq-el-Ahmar (n = 2). Note that moderate heating (200‒500 °C) increases CS to values of 18 to 23 nm (compared to CS ≈ 17 nm in unheated teeth), whereas natural diagenesis decreases CS and increases microstrain. d, Relation between enameloid microstrain and CS of molariform teeth in: fresh unheated fish, fish cooked in the oven (low heat), and fish cooked in the fire (up to 900 °C). The curved stippled red line marks the trend between microstrain and CS. Note that above a temperature of 600 °C the CS, value strongly increases, reaching a maximum value of up to 68 nm in calcinated teeth, while microstrain decreases below a value of 0.20%.
Extended Data Fig. 6 δ18OPO4-δ13C values of ancient and modern Cyprinidae tooth enameloid from Gesher Benot Ya’aqov (GBY), ‘Ubeidiya (UB), Lake Kinneret, and the Jordan River.
a, The phosphate and the carbonate fraction isotope composition (δ18OPO4-δ13C) in fossil L. longiceps teeth from ‘Ubeidiya (1.5 Ma; n = 9) and GBY (0.78 Ma; n = 29), compared with that observed for modern L. longiceps (Lake Kinneret; n = 5), reveal that diagenesis did not alter the fossil teeth and that the isotopic values represent changes in water salinity level and temperatures; b, Quadratic discriminant analysis of the phosphate and the carbonate fraction isotope composition (δ18OPO4-δ13C) in the sampled teeth, in various aquatic habitats: Lake Kinneret (black); Jordan River (light blue) ‘Ubeidiya (orange); GBY A (green) and GBY B (Blue). The ellipse for each habitat is the 95% confidence interval. The dot markers indicate teeth and the “+” marker indicates the multivariate mean of each group. The discrimination diagram shows distinct separation between aquatic habitats due to water evaporation rate and salinity level (Supplemantery Tables 7–8; Wilk’s lambda = 0.033, F = 20.33, p < 0.0001, n = 24).
Supplementary information
Supplementary Information
Supplementary Text A–F (A, the GBY site; B, Cyprinidae pharyngeal teeth; C, natural versus cultural assemblages of GBY fish remains; D, tooth mineral component, XRD analysis; E, spatial analyses of fish remains and burnt flint microartifacts; and F, stable oxygen and carbon isotope analyses of fish teeth), references, Tables 1–8 and Figs. 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zohar, I., Alperson-Afil, N., Goren-Inbar, N. et al. Evidence for the cooking of fish 780,000 years ago at Gesher Benot Ya’aqov, Israel. Nat Ecol Evol 6, 2016–2028 (2022). https://doi.org/10.1038/s41559-022-01910-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01910-z
This article is cited by
-
Ula Thirra: a case study in the geomagnetic detection of combustion features in Channel Country of far south-western Queensland
Archaeological and Anthropological Sciences (2023)
-
Brief interviews with hideous stone: a glimpse into the butchery site of Isernia La Pineta — a combined technological and use-wear approach on the lithic tools to evaluate the function of a Lower Palaeolithic context
Archaeological and Anthropological Sciences (2023)