Abstract
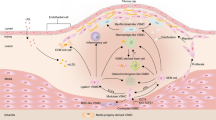
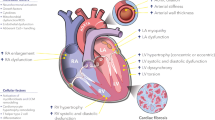
Cardiovascular disease continues to grow as a massive global health burden, with coronary artery disease being one of its most lethal varieties. The pathogenesis of atherosclerosis induces changes in the blood vessel and its extracellular matrix (ECM) in each vascular layer. The alteration of the ECM homeostasis has significant modulatory effects on the inflammatory response, the proliferation and migration of vascular smooth muscle cells, neointimal formation, and vascular fibrosis seen in atherosclerosis. In this literature review, the role of the ECM, the multitude of components, and alterations to these components in the pathogenesis of atherosclerosis are discussed with a focus on versatile cellular phenotypes in the structure of blood vessel. An understanding of the various effects of ECM alterations opens up a plethora of therapeutic options that would mitigate the substantial health toll of atherosclerosis on the global population.



Similar content being viewed by others
References
Benjamin, E. J., et al. (2019). Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation, 139(10), 56–528. https://doi.org/10.1161/CIR.0000000000000659.
Virani, S. S., et al. (2020). Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation, 141(9), 139–596. https://doi.org/10.1161/CIR.0000000000000757.
Ferraz, M., et al. (2012). Correlation of lifetime progress of atherosclerosis and morphologic markers of severity in humans: new tools for a more sensitive evaluation. Clinics, 67(9), 1071–1075. https://doi.org/10.6061/clinics/2012(09)15.
Melly, L., Torregrossa, G., Lee, T., Jansens, J.-L., & Puskas, J. D. (2018). Fifty years of coronary artery bypass grafting. Journal of Thoracic Disease, 10(3), 1960–1967. https://doi.org/10.21037/jtd.2018.02.43.
McKavanagh, P., Yanagawa, B., Zawadowski, G., & Cheema, A. (2017). Management and prevention of saphenous vein graft failure: a review. Cardiol. Ther., 6(2), 203–223. https://doi.org/10.1007/s40119-017-0094-6.
Zhao, Y., Vanhoutte, P. M., & Leung, S. W. S. (2015). Vascular nitric oxide: beyond eNOS. Journal of Pharmacological Sciences, 129(2), 83–94. https://doi.org/10.1016/j.jphs.2015.09.002.
Galley, H. F., & Webster, N. R. (2004). Physiology of the endothelium. British Journal of Anaesthesia, 93(1), 105–113. https://doi.org/10.1093/bja/aeh163.
Marchio, P., Guerra-Ojeda, S., Vila, J. M., Aldasoro, M., Victor, V. M., & Mauricio, M. D. (2019). Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxidative Medicine and Cellular Longevity, 2019, 1–32. https://doi.org/10.1155/2019/8563845.
Langheinrich, A. C., et al. (2006). Correlation of vasa vasorum neovascularization and plaque progression in aortas of apolipoprotein E−/−/low-density lipoprotein−/− double knockout mice. Atherosclerosis, Thrombosis, and Vascular Biology, 26(2), 347–352. https://doi.org/10.1161/01.ATV.0000196565.38679.6d.
Maiellaro, K., & Taylor, W. (2007). The role of the adventitia in vascular inflammation. Cardiovascular Research, 75(4), 640–648. https://doi.org/10.1016/j.cardiores.2007.06.023.
Scott, N. A., et al. (1996). Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation, 93(12), 2178–2187. https://doi.org/10.1161/01.CIR.93.12.2178.
Prado, C. M., Ramos, S. G., Elias, J., & Rossi, M. A. (2008). Turbulent blood flow plays an essential localizing role in the development of atherosclerotic lesions in experimentally induced hypercholesterolaemia in rats: turbulent blood flow and hypercholesterolaemia. International Journal of Experimental Pathology, 89(1), 72–80. https://doi.org/10.1111/j.1365-2613.2007.00564.x.
Shyy, Y. J., Hsieh, H. J., Usami, S., & Chien, S. (1994). Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proceedings of the National Academy of Sciences, 91(11), 4678–4682. https://doi.org/10.1073/pnas.91.11.4678.
Schwenke, D. C., & Carew, T. E. (1989). Initiation of atherosclerotic lesions in cholesterol-fed rabbits. I. Focal increases in arterial LDL concentration precede development of fatty streak lesions. Arterioscler Off J Am Heart Assoc Inc, 9(6), 895–907. https://doi.org/10.1161/01.ATV.9.6.895.
Sorescu, G. P., et al. (2003). Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. The Journal of Biological Chemistry, 278(33), 31128–31135. https://doi.org/10.1074/jbc.M300703200.
Liu, Y., et al. (2002). Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arteriosclerosis, Thrombosis, and Vascular Biology, 22(1), 76–81. https://doi.org/10.1161/hq0102.101822.
Emini Veseli, B., et al. (2017). Animal models of atherosclerosis. European Journal of Pharmacology, 816, 3–13. https://doi.org/10.1016/j.ejphar.2017.05.010.
Miteva, K., Madonna, R., de Caterina, R., & Van Linthout, S. (2018). Innate and adaptive immunity in atherosclerosis. Vascular Pharmacology, 107, 67–77.
Libby, P., & Hansson, G. K. (2015). Inflammation and immunity in diseases of the arterial tree: players and layers. Circulation Research, 116(2), 307–311. https://doi.org/10.1161/CIRCRESAHA.116.301313.
Libby, P. (2013). History of discovery: inflammation in atherosclerosis (p. 15).
Wang, Y., et al. (2019). Smooth muscle cells contribute the majority of foam cells in ApoE (apolipoprotein E)-deficient mouse atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(5), 876–887. https://doi.org/10.1161/ATVBAHA.119.312434.
Chistiakov, D. A., Melnichenko, A. A., Myasoedova, V. A., Grechko, A. V., & Orekhov, A. N. (2017). Mechanisms of foam cell formation in atherosclerosis. J Moecular Med, 95(11), 1153–1165.
Taleb, S. (2016). Inflammation in atherosclerosis. Archives of Cardiovascular Diseases, 109(12), 708–715. https://doi.org/10.1016/j.acvd.2016.04.002.
Ketelhuth, D. F. J., & Hansson, G. K. (2016). Adaptive response of T and B cells in atherosclerosis. Circulation Research, 118(4), 668–678. https://doi.org/10.1161/CIRCRESAHA.115.306427.
Tabas, I., & Lichtman, A. H. (2017). Monocyte-macrophages and T cells in atherosclerosis. Immunity, 47(4), 621–634. https://doi.org/10.1016/j.immuni.2017.09.008.
Xu, J., & Shi, G.-P. (2014). Vascular wall extracellular matrix proteins and vascular diseases. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1842(11), 2106–2119. https://doi.org/10.1016/j.bbadis.2014.07.008.
Ponticos, M., & Smith, B. D. (2014). Extracellular matrix synthesis in vascular disease: hypertension, and atherosclerosis. Journal of Biomedical Research, 28(1), 25–39. https://doi.org/10.7555/JBR.27.20130064.
Eble, J. A., & Niland, S. (2009). The extracellular matrix of blood vessels. Curr Pharaceutical Des., 15(12), 1385–1400.
Katsuda, S., & Kaji, T. (2003). Atherosclerosis and extracellular matrix. Journal of Atherosclerosis and Thrombosis, 10(5), 267–274. https://doi.org/10.5551/jat.10.267.
Rhodes, J. M., & Simons, M. (2007). The extracellular matrix and blood vessel formation: not just a scaffold. Journal of Cellular and Molecular Medicine, 11(2), 176–205. https://doi.org/10.1111/j.1582-4934.2007.00031.x.
Humphrey, J. D., Dufresne, E. R., & Schwartz, M. A. (2014). Mechanotransduction and extracellular matrix homeostasis. Nature Reviews. Molecular Cell Biology, 15(12), 802–812. https://doi.org/10.1038/nrm3896.
Nissen, R., Cardinale, G. J., & Udenfriend, S. (1978). Increased turnover of arterial collagen in hypertensive rats. Proceedings of the National Academy of Sciences, 75(1), 451–453. https://doi.org/10.1073/pnas.75.1.451.
Prajapati, R. T., Chavally-Mis, B., Herbage, D., Eastwood, M., & Brown, R. A. (2000). Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair and Regeneration, 8(3), 226–237.
Heeneman, S., et al. (2003). The dynamic extracellular matrix: intervention strategies during heart failure and atherosclerosis: the dynamic extracellular matrix. The Journal of Pathology, 200(4), 516–525. https://doi.org/10.1002/path.1395.
Simionescu, D., et al. (2006). Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials, 27(5), 702–713.
Halka, A. T., et al. (2008). The effects of stretch on vascular smooth muscle cell phenotype in vitro. Cardiovascular Pathology, 17(2), 98–102.
Smith, E. B. (1965). The influence of age and atherosclerosis on the chemistry of aortic intima: part 2. Collagen and mucopolysaccharides. Journal of Atherosclerosis Research, 5(2), 241–248.
Adiguzel, E., Ahmad, P. J., Franco, C., & Bendeck, M. P. (2009). Collagens in the progression and complications of atherosclerosis. Vascular Medicine, 14(1), 73–89. https://doi.org/10.1177/1358863X08094801.
Liu, B., Itoh, H., Louie, O., Kubota, K., & Kent, K. C. (2004). The role of phospholipase C and phosphatidylinositol 3-kinase in vascular smooth muscle cell migration and proliferation. The Journal of Surgical Research, 120(2), 256–265.
Hou, G., Mulholland, D., Gronska, M. A., & Bendeck, M. P. (2000). Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinase synthesis after arterial injury. The American Journal of Pathology, 156(2), 467–476. https://doi.org/10.1016/S0002-9440(10)64751-7.
Steffensen, L. B., & Rasmussen, L. M. (2018). A role for collagen type IV in cardiovascular disease? American Journal of Physiology. Heart and Circulatory Physiology, 315(3), H610–H625. https://doi.org/10.1152/ajpheart.00070.2018.
Jones, P. L., Jones F. S., Zhou B., & Rabinovitch M. (1999). Induction of vascular smooth muscle cell tenascin-C gene expression by denatured type I collagen is dependent upon a β3 integrin-mediated mitogen-activated protein kinase pathway and a 122-base pair promoter element. Journal of Cell Science, 112(4), 435–445.
Chistiakov, D. A., Sobenin, I. A., & Orekhov, A. N. (2013). Vascular extracellular matrix in atherosclerosis. Cardiology in Review, 21(6), 270–288. https://doi.org/10.1097/CRD.0b013e31828c5ced.
Rekhter, M. D., et al. (2000). Hypercholesterolemia causes mechanical weakening of rabbit atheroma: local collagen loss as a prerequisite of plaque rupture. Circulation Research, 86(1), 101–108. https://doi.org/10.1161/01.RES.86.1.101.
Faia, K. L., Davis, W. P., Marone, A. J., & Foxall, T. L. (2002). Matrix metalloproteinases and tissue inhibitors of metalloproteinases in hamster aortic atherosclerosis: correlation with in-situ zymography. Atheroscler. J., 160(2), 325–337.
Gayral, S., et al. (2014). Elastin-derived peptides potentiate atherosclerosis through the immune Neu1–PI3Kγ pathway. Cardiovascular Research, 102(1), 118–127. https://doi.org/10.1093/cvr/cvt336.
Bültmann, A., Li, Z., Wagner, S., Gawaz, M., Ungerer, M., & Münch, G. (2010). Impact of glycoprotein VI and platelet adhesion on atherosclerosis—a possible role of fibronectin. Journal of Molecular and Cellular Cardiology, 49(3), 532–542.
Viola, M., et al. (2016). Extracellular matrix in atherosclerosis: hyaluronan and proteoglycans insights. Current Medicinal Chemistry, 23(26), 2958–2971.
Ilhan, F. (2015). Atherosclerosis and the role of immune cells. World Journal of Clinical Cases, 3(4), 345. https://doi.org/10.12998/wjcc.v3.i4.345.
Arroyo, A. G., & Iruela-Arispe, M. L. (2010). Extracellular matrix, inflammation, and the angiogenic response. Cardiovascular Research, 86(2), 226–235. https://doi.org/10.1093/cvr/cvq049.
Stringa, E., Knäuper, V., Murphy, G., & Gavrilovic, J. (2000). Collagen degradation and platelet-derived growth factor stimulate the migration of vascular smooth muscle cells. Journal of Cell Science, 113, 2055–2064.
Barillari, G., Albonici, L., Incerpi, S., Volpi, A., Ensoli, B., & Manzari, V. (2001). Inflammatory cytokines stimulate vascular smooth muscle cells locomotion and growth by enhancing α5β1 integrin expression and function. Atherosclerosis, 154(2), 377–385.
Galis, Z. S., Muszynski, M., Sukhova, G. K., Simon-Morrissey, E., & Libby, P. (1994). Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Annals of the New York Academy of Sciences, 748(1), 501–507.
Ghesquiere, S. A. I., et al. (2005). Macrophage-specific overexpression of group IIa sPLA 2 increases atherosclerosis and enhances collagen deposition. Journal of Lipid Research, 46(2), 201–210. https://doi.org/10.1194/jlr.M400253-JLR200.
Abdulhussein, R., McFadden, C., Fuentes-Prior, P., & Vogel, W. F. (2004). Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. The Journal of Biological Chemistry, 279(30), 31462–31470. https://doi.org/10.1074/jbc.M400651200.
Dunér, P., et al. (2011). Immunization of apoE–/– mice with aldehyde-modified fibronectin inhibits the development of atherosclerosis. Cardiovascular Research, 91(3), 528–536. https://doi.org/10.1093/cvr/cvr101.
Caligiuri, G., et al. (2006). Reduced immunoregulatory CD31 + T cells in patients with atherosclerotic abdominal aortic aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(3), 618–623. https://doi.org/10.1161/01.ATV.0000200380.73876.d9.
Shankavaram, U. T., et al. (2001). Monocyte membrane type 1-matrix metalloproteinase: prostaglandin-dependent regulation and role in metalloproteinase-2 activation. The Journal of Biological Chemistry, 276(22), 19027–19032. https://doi.org/10.1074/jbc.M009562200.
Sibinga, N. E. S., et al. (1997). Collagen VIII is expressed by vascular smooth muscle cells in response to vascular injury. Circulation Research, 80(4), 532–541.
Xu, H., Jiang, J., Chen, W., Li, W., & Chen, Z. (2019). Vascular macrophages in atherosclerosis. Journal of Immunology Research, 2019, 1–14. https://doi.org/10.1155/2019/4354786.
Marom, B., Rahat, M. A., Lahat, N., Weiss-Cerem, L., Kinarty, A., & Bitterman, H. (2007). Native and fragmented fibronectin oppositely modulate monocyte secretion of MMP-9. Journal of Leukocyte Biology, 81(6), 1466–1476. https://doi.org/10.1189/jlb.0506328.
Ricard-Blum, S., & Salza, R. (2014). Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Experimental Dermatology, 23(7), 457–463. https://doi.org/10.1111/exd.12435.
Payne, G. A., et al. (2017). The matrikine proline-glycine-proline is locally generated following acute arterial vascular injury. Circulation, 136(1), 21106.
Liu, N. et al. (2001). Metastatin: a hyaluronan-binding complex from cartilage that inhibits tumor growth. Cancer Research, 61(3), 1022–1028.
Wells, J. M., Gaggar, A., & Blalock, J. E. (2015). MMP generated matrikines. Matrix Biology, 44–46, 122–129. https://doi.org/10.1016/j.matbio.2015.01.016.
Assadian, S., et al. (2012). p53 inhibits angiogenesis by inducing the production of Arresten. Cancer Research, 72(5), 1270–1279. https://doi.org/10.1158/0008-5472.CAN-11-2348.
Okada, M., & Yamawaki, H. (2019). A current perspective of canstatin, a fragment of type IV collagen alpha 2 chain. Journal of Pharmacological Sciences, 139(2), 59–64. https://doi.org/10.1016/j.jphs.2018.12.001.
Walia, A., Yang, J. F., Huang, Y., Rosenblatt, M. I., Chang, J.-H., & Azar, D. T. (2015). Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochimica et Biophysica Acta (BBA) - General Subjects, 1850(12), 2422–2438. https://doi.org/10.1016/j.bbagen.2015.09.007.
Neve, A., Cantatore, F. P., Maruotti, N., Corrado, A., & Ribatti, D. (2014). Extracellular matrix modulates angiogenesis in physiological and pathological conditions. BioMed Research International, 2014, 1–10. https://doi.org/10.1155/2014/756078.
Zhao, Y., Gu, X., Zhang, N., Kolonin, M. G., An, Z., & Sun, K. (2016). Divergent functions of endotrophin on different cell populations in adipose tissue. American Journal of Physiology. Endocrinology and Metabolism, 311(6), E952–E963. https://doi.org/10.1152/ajpendo.00314.2016.
Väisänen, M.-R., Väisänen, T., Tu, H., Pirilä, P., Sormunen, R., & Pihlajaniemi, T. (2006). The shed ectodomain of type XIII collagen associates with the fibrillar fibronectin matrix and may interfere with its assembly in vitro. Biochemical Journal, 393(1), 43–50. https://doi.org/10.1042/BJ20051073.
Jones, V. A., Patel, P. M., Gibson, F. T., Cordova, A., & Amber, K. T. (2020). The role of collagen XVII in cancer: squamous cell carcinoma and beyond. Frontiers in Oncology, 10, 352. https://doi.org/10.3389/fonc.2020.00352.
Mao, W., et al. (2010). Evaluation of recombinant endostatin in the treatment of atherosclerotic plaques and neovascularization in rabbits. Journal of Zhejiang University. Science. B, 11(8), 599–607. https://doi.org/10.1631/jzus.B1001011.
Kojima, T., Azar, D. T., & Chang, J.-H. (2008). Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Letters, 582(17), 2515–2520. https://doi.org/10.1016/j.febslet.2008.06.014.
Stine, J. M., Sun, Y., Armstrong, G., Bowler, B. E., & Briknarová, K. (2015). Structure and unfolding of the third type III domain from human fibronectin. Biochemistry, 54(44), 6724–6733. https://doi.org/10.1021/acs.biochem.5b00818.
Douglass, S., Goyal, A., & Iozzo, R. V. (2015). The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connective Tissue Research, 56(5), 381–391. https://doi.org/10.3109/03008207.2015.1045297.
Wanga, S., et al. (2017). Aortic microcalcification is associated with elastin fragmentation in Marfan syndrome: microcalcification and elastin fragmentation in Marfan syndrome. The Journal of Pathology, 243(3), 294–306. https://doi.org/10.1002/path.4949.
Wang, D., Wang, Z., Zhang, L., & Wang, Y. (2017). Roles of cells from the arterial vessel wall in atherosclerosis. Mediators of Inflammation, 2017, 1–9. https://doi.org/10.1155/2017/8135934.
Gimbrone, M. A., & García-Cardeña, G. (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circulation Research, 118(4), 620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301.
Stöllberger, C., & Finsterer, J. (2002). Role of infectious and immune factors in coronary and cerebrovascular arteriosclerosis. Clinical and Vaccine Immunology, 9(2), 207–215. https://doi.org/10.1128/CDLI.9.2.207-215.2002.
Halper, J. (2018). Basic components of vascular connective tissue and extracellular matrix. In Advances in Pharmacology (Vol. 81, pp. 95–127). Amsterdam: Elsevier.
Pavlovic, S., et al. (Feb. 2006). Targeting prostaglandin E 2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. The Journal of Biological Chemistry, 281(6), 3321–3328. https://doi.org/10.1074/jbc.M506846200.
Milutinović, A., Šuput, D., & Zorc-Pleskovič, R. (2019). Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: an updated review. Bosnian Journal of Basic Medical Sciences, 20(1), 21–30. https://doi.org/10.17305/bjbms.2019.4320.
Basatemur, G. L., Jørgensen, H. F., Clarke, M. C. H., Bennett, M. R., & Mallat, Z. (2019). Vascular smooth muscle cells in atherosclerosis. Nature Reviews. Cardiology, 16, 727–744.
Chen, Z., Fu, Y., & Kong, W. (2015). Extracellular matrix on the phenotypic switching of vascular smooth muscle cells. Current Angiogenesis, 4(1), 46–59. https://doi.org/10.2174/221155280401160517170529.
Kingsley, K., et al. (2002). ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochemical and Biophysical Research Communications, 293(3), 1000–10006.
Rensen, S. S. M., Doevendans, P. A. F. M., & van Eys, G. J. J. M. (2007). Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Heart Journal, 15(3), 100–108. https://doi.org/10.1007/BF03085963.
Pickering, J. G., et al. (2000). α5β1 integrin expression and luminal edge fibronectin matrix assembly by smooth muscle cells after arterial injury. The American Journal of Pathology, 156(2), 453–465. https://doi.org/10.1016/S0002-9440(10)64750-5.
Cheng, J., et al. (2007). Mechanical stretch inhibits oxidized low density lipoprotein-induced apoptosis in vascular smooth muscle cells by up-regulating integrin Vbeta3 and stabilization of PINCH-1. The Journal of Biological Chemistry, 282(47), 34268–34275. https://doi.org/10.1074/jbc.M703115200.
Doran, A. C., Meller, N., & McNamara, C. A. (2008). Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(5), 812–819. https://doi.org/10.1161/ATVBAHA.107.159327.
Allahverdian, S., Chaabane, C., Boukais, K., Francis, G. A., & Bochaton-Piallat, M.-L. (2018). Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovascular Research, 114(4), 540–550. https://doi.org/10.1093/cvr/cvy022.
O’Brien, K. D., et al. (1993). Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. The Journal of Clinical Investigation, 92(2), 945–951. https://doi.org/10.1172/JCI116670.
Bennett, M. R., Sinha, S., & Owens, G. K. (2016). Vascular smooth muscle cells in atherosclerosis. Circulation Research, 118(4), 692–702. https://doi.org/10.1161/CIRCRESAHA.115.306361.
Roy, J., et al. (2002). Fibronectin promotes cell cycle entry in smooth muscle cells in primary culture. Experimental Cell Research, 273(2), 169–177.
Williams, E. S., Wilson, E., & Ramos, K. S. (2012). NF-B and matrix-dependent regulation of osteopontin promoter activity in allylamine-activated vascular smooth muscle cells. Oxidative Medicine and Cellular Longevity, 2012, 1–10. https://doi.org/10.1155/2012/496540.
Bentzon, J. F., Otsuka, F., Virmani, R., & Falk, E. (2014). Mechanisms of plaque formation and rupture. Circulation Research, 114(12), 1852–1866. https://doi.org/10.1161/CIRCRESAHA.114.302721.
Singh, & Torzewski. (2019). Fibroblasts and their pathological functions in the fibrosis of aortic valve sclerosis and atherosclerosis. Biomolecules, 9(9), 472. https://doi.org/10.3390/biom9090472.
Holm Nielsen, S., et al. (2018). A biomarker of collagen type I degradation is associated with cardiovascular events and mortality in patients with atherosclerosis. Jounral Intern. Med., 285(1), 118–123.
Holm Nielsen, S., et al. (2018). Markers of basement membrane remodeling are associated with higher mortality in patients with known atherosclerosis. Journal of the American Heart Association, 7(21), e009193. https://doi.org/10.1161/JAHA.118.009193.
Bertelsen, D. M., et al. (2018). Matrix metalloproteinase mediated type I collagen degradation is an independent predictor of increased risk of acute myocardial infarction in postmenopausal women. Scientific Reports, 8(1), 5371. https://doi.org/10.1038/s41598-018-23458-4.
Otaki, Y., et al. (2016). Serum carboxy-terminal telopeptide of type I collagen (I-CTP) is predictive of clinical outcome in peripheral artery disease patients following endovascular therapy. Heart and Vessels, 32, 14–156.
Holm Nielsen, S., et al. (2020). Exploring the role of extracellular matrix proteins to develop biomarkers of plaque vulnerability and outcome. Journal of Internal Medicine, 287(5), 493–513. https://doi.org/10.1111/joim.13034.
Garvin, P., Jonasson, L., Nilsson, L., Falk, M., & Kristenson, M. (2015). Plasma matrix metalloproteinase-9 levels predict first-time coronary heart disease: an 8-year follow-up of a community-based middle aged population. PLoS ONE, 10(9), e0138290. https://doi.org/10.1371/journal.pone.0138290.
Kai, H., et al. (1998). Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. Journal of the American College of Cardiology, 32(2), 368–372. https://doi.org/10.1016/S0735-1097(98)00250-2.
Webb, K. E., Henney, A. M., Anglin, S., Humphries, S. E., & McEwan, J. R. (1997). Expression of matrix metalloproteinases and their inhibitor TIMP-1 in the rat carotid artery after balloon injury. Arteriosclerosis, Thrombosis, and Vascular Biology, 17(9), 1837–1844. https://doi.org/10.1161/01.ATV.17.9.1837.
Roycik, M., Myers, J., Newcomer, R., & Sang, Q. (2013). Matrix metalloproteinase inhibition in atherosclerosis and stroke. Current Molecular Medicine, 13(8), 1299–1313.
Prescott, M. F., et al. (2006). Effect of matrix metalloproteinase inhibition on progression of atherosclerosis and aneurysm in LDL receptor-deficient mice overexpressing MMP-3, MMP-12, and MMP-13 and on restenosis in rats after balloon injury. Annals of the New York Academy of Sciences, 878(1), 179–190.
Ruddy, J. M., Ikonomidis, J. S., & Jones, J. A. (2016). Multidimensional contribution of matrix metalloproteinases to atherosclerotic plaque vulnerability: multiple mechanisms of inhibition to promote stability. Journal of Vascular Research, 53(1–2), 1–16. https://doi.org/10.1159/000446703.
Luan, Z., Chase, A. J., & Newby, A. C. (2003). Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology, 23(5), 769–775. https://doi.org/10.1161/01.ATV.0000068646.76823.AE.
Brown, D. L., Desai, K. K., Vakili, B. A., Nouneh, C., Lee, H.-M., & Golub, L. M. (2004). Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(4), 733–738. https://doi.org/10.1161/01.ATV.0000121571.78696.dc.
Axisa, B., et al. (2002). Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke, 33(12), 2858–2864. https://doi.org/10.1161/01.STR.0000038098.04291.F6.
Aikawa, M., et al. (1998). Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation, 97(24), 2433–2444. https://doi.org/10.1161/01.CIR.97.24.2433.
Crisby, M., Nordin-Fredriksson, G., Shah, P. K., Yano, J., Zhu, J., & Nilsson, J. (2001). Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation, 103(7), 926–933. https://doi.org/10.1161/01.CIR.103.7.926.
Lutgens, E., et al. (2002). Transforming growth factor-β mediates balance between inflammation and fibrosis during plaque progression. Arteriosclerosis, Thrombosis, and Vascular Biology, 22(6), 975–982. https://doi.org/10.1161/01.ATV.0000019729.39500.2F.
Wang, N., et al. (2010). Role of TGF-β1 in bone matrix production in vascular smooth muscle cells induced by a high-phosphate environment. Nephron. Experimental Nephrology, 115, 60–68.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author DKA has received grants from the National Institutes of Health. The other authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants and/or animals performed by any of the authors.
No writing assistance was utilized in the production of this manuscript.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohindra, R., Agrawal, D.K. & Thankam, F.G. Altered Vascular Extracellular Matrix in the Pathogenesis of Atherosclerosis. J. of Cardiovasc. Trans. Res. 14, 647–660 (2021). https://doi.org/10.1007/s12265-020-10091-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10091-8